Questions | Answer | Explanations |
31 Which list of elements consists of metalloids, only?
(1) B, Al, Ga (3) O, S, Se
(2) C, N, P (4) Si, Ge, As | 4 | staircase, NOT Al |
 | 1 | must have same atomic number but different mass number |
33 Which general trend is found in Period 2 on the Periodic Table as the elements are considered in order of increasing atomic number?
(1) decreasing atomic mass
(2) decreasing electronegativity
(3) increasing atomic radius
(4) increasing first ionization energy | 4 | see Table S |
34 What is the gram-formula mass of Ca3(PO4)2?
(1) 248 g/mol (3) 279 g/mol
(2) 263 g/mol (4) 310. g/mol | 4 | (3x40) + (2x31.0)+ (8x16.0) |
35 What is the total number of pairs of electrons shared between the carbon atom and the oxygen atom in a molecule of methanal?
(1) 1 (3) 3
(2) 2 (4) 4 | 2 | carbon and oxygen have a double bond 2 pairs of electrons |
36 When sodium and fluorine combine to produce the compound NaF, the ions formed have the same electron configuration as atoms of
(1) argon, only
(2) neon, only
(3) both argon and neon
(4) neither argon nor neon | 2 | both end up with 10 electrons |
37 In which compound is the ratio of metal ions to nonmetal ions 1 to 2?
(1) calcium bromide (3) calcium phosphide
(2) calcium oxide (4) calcium sulfide | 1 | CaBr2 |
38 What is the concentration of O2(g), in parts per million, in a solution that contains 0.008 gram of O2(g) dissolved in 1000. grams of H2O(l)?
(1) 0.8 ppm (3) 80 ppm
(2) 8 ppm (4) 800 ppm | 2 | 0.008/1,000.008 x 1,000,000 |
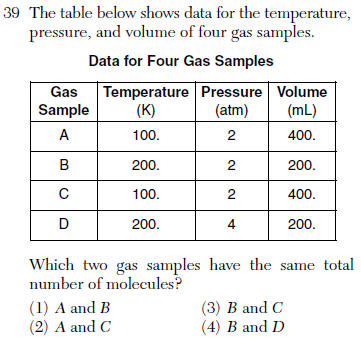 | 2 | same condition, with the same volumes Avogadro's hypothesis |
40 At which temperature is the vapor pressure of ethanol equal to the vapor pressure of propanone at 35°C?
(1) 35°C (3) 82°C
(2) 60.°C (4) 95°C | 2 | 54kpa; ethanol would need a temperature of 60C |

