Questions
Explanations
(1) 298 K (3) 27°C
(2) 267 K (4) 12°C
highest temperature
(1) a tenfold increase
(2) a tenfold decrease
(3) a hundredfold increase
(4) a hundredfold decrease
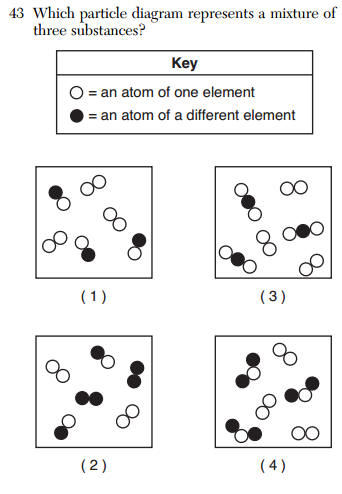
![]()
Which statement describes this system?
(1) The concentration of PCl5(g) is increasing.
(2) The concentration of PCl5(g) is decreasing.
3) The concentrations of PCl5(g) and PCl3(g) are equal.
(4) The concentrations of PCl5(g) and PCl3(g) are constant.
Concentrations are constant and the rates are equal
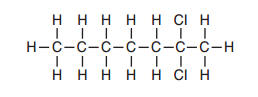
What is the IUPAC name of this compound?
(1) 2-chloroheptane (2) 6-chloroheptane
(3) 2,2-dichloroheptane (4) 6,6-dichloroheptane