0.0ml of NaOH 50.0mL 0.150M HCl pH=-log0.150M pH= 0.82 |
25.0ml of NaOH to 50.0mL of HCl Moles H+=50.0mL x 0.150M=7.50mmol Moles OH-=25.0mL x 0.150M=3.75mmol | | H+ + | OH- | è H2O | | I | 7.50mmol | 3.75mmol | | | C | -3.75mmol | -3.75mmol | | | E | 3.75mmol | 0 | |
| [H+]= | 3.75mmol | =0.05M | | 25.0mL +50.0mL |
pH=-log[H+] pH=-log[0.05M]=0.82
|
49.9ml of NaOH to 50.0mL of HCl Moles H+=50.0mL x 0.150M=7.50mmol Moles OH-=49.9mL x 0.150M=7.485mmol | | H+ + | OH- | è H2O | | I | 7.50mmol | 7.485mmol | | | C | -7.485mmol | -7.485mmol | | | E | 0.015mmol | 0 | |
| [H+]= | 0.015mmol | =0.000 15M | | 49.9mL +50.0mL |
pH=-log[H+] pH=-log[0.000 15M]=3.82
|
50.0ml of NaOH to 50.0mL of HCl Moles H+=50.0mL x 0.150M=7.50mmol Moles OH-=50.0mL x 0.150M=7.50mmol | | H+ + | OH- | è H2O | | I | 7.50mmol | 7.50mmol | | | C | -7.50mmol | -7.50mmol | | | E | 0 | 0 | |
neutralization pH=7 |
50.1ml of NaOH to 50.0mL of HCl Moles H+=50.0mL x 0.150M=7.50mmol Moles OH-=50.1mL x 0.150M=7.515mmol | | H+ + | OH- | è H2O | | I | 7.50mmol | 7.515mmol | | | C | -7.50mmol | -7.50mmol | | | E | 0 | 0.015mmol | |
| [OH- ]= | 0.015mmol | =0.000 15M | | 50.1mL +50.0mL |
pOH=-log[OH-] pOH=-log[0.000 15M]=3.82 pH=14-3.82=10.18
|
75.0ml of NaOH to 50.0mL of HCl Moles H+=50.0mL x 0.150M=7.50mmol Moles OH-=75.0mL x 0.150M=11.25mmol | | H+ + | OH- | è H2O | | I | 7.50mmol | 11.25mmol | | | C | -7.50mmol | -7.50mmol | | | E | 0 | 3.75mmol | |
| [OH- ]= | 3.75mmol | =0.030M | | 75.0mL +50.0mL |
pOH=-log[OH-] pOH=-log[0.030M]=1.52 pH=14-1.52=12.48 |
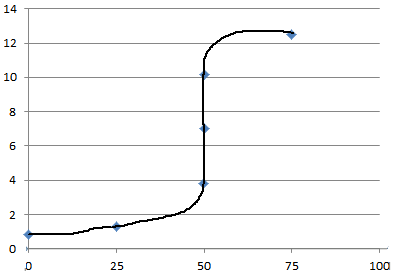
pH | Vol. NaOH | 0.82 | 0.0mL | 1.30 | 25.0mL | 3.82 | 49.9mL | 7.00 | 50.0mL | 10.18 | 50.1mL | 12.48 | 75.0ml |
|  |
|

