Another really handy rearrangement of the ideal gas equation can be used to find the molecular weight of an unknown gas 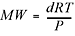 . You’ll get a chance to practice using these in the problems at the end of the chapter. However, there is no need to memorize these last equations since they are all rearrangements of the ideal gas law. Okay, two more important laws and then we’re finished with our discussion of gases, and we move on to solutions. . You’ll get a chance to practice using these in the problems at the end of the chapter. However, there is no need to memorize these last equations since they are all rearrangements of the ideal gas law. Okay, two more important laws and then we’re finished with our discussion of gases, and we move on to solutions. So, for gases, we speak of "standard gas density." This is the density of the gas (expressed in grams per liter) at STP. If you discuss gas density at any other set of conditions, you drop the word standard and specify the pressure and temperature. Also, when you say "standard gas density," you do not need to add "at STP." STP is part of the definition of the term. It does no harm to say "standard gas density at STP," it just looks a bit silly. |

