| Questions | Answer | Explanation |
1 Which of these phrases best describes an atom? (1) a positive nucleus surrounded by a hard negative shell(2) a positive nucleus surrounded by a cloud of negative charges(3) a hard sphere with positive particles uniformly embedded(4) a hard sphere with negative particles uniformly embedded | 2 | nucleus is positive and the negative electrons are somewhere in the cloud outside this nucleus |
2 Which statement is true about a proton and an electron?(1) They have the same masses and the same charges.(2) They have the same masses and different charges.(3) They have different masses and the same charges.(4) They have different masses and different charges. | 4 | protons and neutrons have the same mass electrons are 1/1836 of a proton in mass proton + ;electron - ;neutron no charge |
3 The atomic mass of an element is the weighted average of the masses of (1) its two most abundant isotopes (2) its two least abundant isotopes (3) all of its naturally occurring isotopes (4) all of its radioactive isotopes | 3 | DEFINITION OF AN ISOTOPE all the isotopes |
4 What determines the order of placement of the elements on the modern Periodic Table?(1) atomic number (2) atomic mass (3) the number of neutrons, only (4) the number of neutrons and protons | 1 | PT base on atomic numbers |
5 Which compound contains only covalent bonds? (1) NaOH (3) Ca(OH) 2(2) Ba(OH) 2 (4) CH3OH | 4 | Look for all nonmetals (usually an organic compound) |
6 At 298 K, oxygen (O 2) and ozone (O3) have different properties because their(1) atoms have different atomic numbers (2) atoms have different atomic masses (3) molecules have different molecular structures (4) molecules have different average kinetic energies | 3 | different numbers of atoms = different structures |
7 Which substance represents a compound? (1) C(s) (3) CO(g) (2) Co(s) (4) O 2(g) | 3 | two Capital lettersCompounds are made of 2 or more different elements |
8 All chemical reactions have a conservation of (1) mass, only (2) mass and charge, only (3) charge and energy, only (4) mass, charge, and energy | 4 | everything should be conserved in a reaction |
9 Which characteristic is a property of molecular substances?(1) good heat conductivity (2) good electrical conductivity (3) low melting point (4) high melting point | 3 | molecular is referring to a covalent compound low MP & BP, poor conductors of heat and electricity, nonelectrolytes, brittle |
10 Given the Lewis electron-dot diagram: 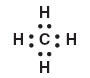
Which electrons are represented by all of the dots? (1) the carbon valence electrons, only (2) the hydrogen valence electrons, only (3) the carbon and hydrogen valence electrons (4) all of the carbon and hydrogen electrons | 3 | dots represent valence electrons that each atom brings into the bond |