| Questions | Answers | Explanations |
41 Based on intermolecular forces, which of these substances would have the highest boiling point?(1) He (3) CH 4(2) O 2 (4) NH3 | 4 | NH3 is the only polar molecules the others are nonpolar |
42 How much heat energy must be absorbed to completely melt 35.0 grams of H2O(s) at 0°C?(1) 9.54 J (3) 11 700 J (2) 146 J (4) 79 100 J | 3 | q=mHf q=35g x 334J/g |
43 The graph below represents the uniform heating of a substance, starting below its melting point, when the substance is solid.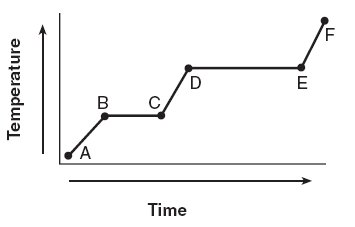
Which line segments represent an increase in average kinetic energy?(1) AB and BC (3) BC and DE(2) and AB CD (4) DE and EF | 2 | Temperature is Average Kinetic Energy increasing KE occurs in the diagonal segments |
| 44 Given the three organic structural formulas shown below: 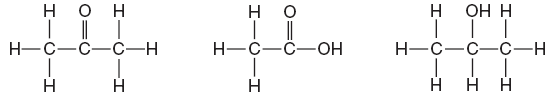
Which organic-compound classes are represented by these structural formulas, as shown from left to right? (1) ester, organic acid, ketone (2) ester, aldehyde, organic acid (3) ketone, aldehyde, alcohol (4) ketone, organic acid, alcohol | 4 | Use table R |
45 Given the reaction at equilibrium: N 2(g) + O2(g) + energy <--> 2 NO(g)Which change will result in a decrease in the amount of NO(g) formed?(1) decreasing the pressure (2) decreasing the concentration of N 2(g)(3) increasing the concentration of O 2(g)(4) increasing the temperature | 2 | since the moles of gases are the same pressure changes do nothing raising the temperature is adding energy and will shift the equilibrium right thus increasing NO |
46 Given the equation: X + Cl2 --> C2H5Cl + HClWhich molecule is represented by X?(1) C 2H4 (3) C3H6(2) C 2H6 (4) C3H8 | 2 | find the missing piece |
47 Which metal reacts spontaneously with a solution containing zinc ions?(1) magnesium (3) copper (2) nickel (4) silver | 1 | Table J above zinc |
48 Which statement correctly describes a solution with a pH of 9?(1) It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue.(2) It has a higher concentration of OH – than H3O+ and causes litmus to turn blue.(3) It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow.(4) It has a higher concentration of OH – than H3O+ and causes methyl orange to turn red. | 2 | pH of 9 is basic so OH- has a larger concentration than H3O+ litmus is blue in a base using Table M acid-bases |
49 How many days are required for 200. grams of radon-222 to decay to 50.0 grams?(1) 1.91 days (3) 7.64 days (2) 3.82 days (4) 11.5 days | 3 | 200->100->50 2 (1/2 lives) 2 x 3.82d per half life |
50 A student calculates the density of an unknown solid. The mass is 10.04 grams, and the volume is 8.21 cubic centimeters. How many significant figures should appear in the final answer?(1) 1 (3) 3 (2) 2 (4) 4 | 3 | since division is involved, the answer is only as precise as the least precise measurement, which is 8.21 so the answer has 3 sig figs |