Questions | Answer | Explanations |
11 Which type of bonding is found in all molecular substances?
(1) covalent bonding (3) ionic bonding
(2) hydrogen bonding (4) metallic bonding | 1 | molecular is covalent |
12 An aqueous solution of sodium chloride is best classified as a
(1) homogeneous compound
(2) homogeneous mixture
(3) heterogeneous compound
(4) heterogeneous mixture | 2 | solution is homogeneous |
13 What is the total number of electrons shared in a double covalent bond between two atoms?
(1) 1 (3) 8
(2) 2 (4) 4 | 4 | 2 electrons = 1 bond |
14 Which formula represents a nonpolar molecule?
(1) H2S (3) CH4
(2) HCl (4) NH3 | 3 | draw them, nonpolar is symmetrical |
15 What occurs when an atom loses an electron?
(1) The atomís radius decreases and the atom becomes a negative ion.
(2) The atomís radius decreases and the atom becomes a positive ion.
(3) The atomís radius increases and the atom becomes a negative ion.
(4) The atomís radius increases and the atom becomes a positive ion. | 2 | lose electron become positive and decrease in size |
16 Two samples of gold that have different temperatures are placed in contact with one another. Heat will flow spontaneously from a sample of gold at 60įC to a sample of gold that has a temperature of
(1) 50įC (3) 70įC
(2) 60įC (4) 80įC | 1 | heat flows from hot to cold |
17 Under which conditions of temperature and pressure would helium behave most like an ideal gas?
(1) 50 K and 20 kPa (3) 750 K and 20 kPa
(2) 50 K and 600 kPa (4) 750 K and 600 kPa | 3 | ideal gas=ideal vacation high temp and low pressure |
18 A sample of oxygen gas is sealed in container X. A sample of hydrogen gas is sealed in container Z. Both samples have the same volume, temperature,
and pressure. Which statement is true?
(1) Container X contains more gas molecules than container Z.
(2) Container X contains fewer gas molecules than container Z.
(3) Containers X and Z both contain the same number of gas molecules.
(4) Containers X and Z both contain the same mass of gas. | 3 | same volume under the same conditions same number of molecules Avagodro's Hypothesis |
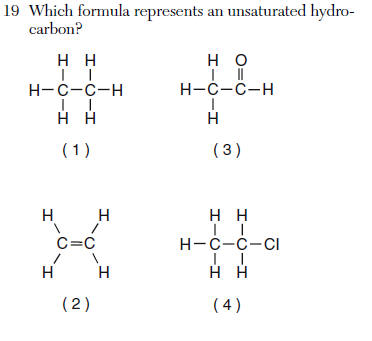 | 2 | unsaturated=double or triple bonded hydrocarbon= only hydrogen and carbon |
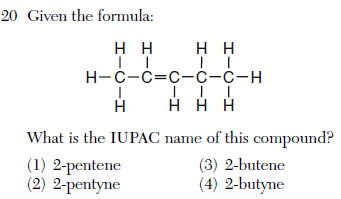 | 1 | 5 carbons pent- double bond -ene at the second carbon 2- |

