Questions | Answer | Explanations |
31 The percentage by mass of Br in the compound
AlBr3 is closest to
(1) 10.% (3) 75%
(2) 25% (4) 90.% | 4 | (3(79.9) /(3(79.9) +27)) x 100% |
32 Which symbol represents a particle with a total of 10 electrons?
(1) N (3) Al
(2) N3+ (4) Al3+ | 4 | Al = 13 electrons Al3+ loses 3 electrons |
33 Which electron configuration represents an atom of aluminum in an excited state?
(1) 2-7-4 (3) 2-8-3
(2) 2-7-7 (4) 2-8-6 | 1 | Al = 13 electrons promote 1 electron 2-8-3 ==> 2-7-4 |
34 At STP, an element that is a brittle solid and a poor conductor of heat and electricity could have an atomic number of
(1) 12 (3) 16
(2) 13 (4) 17 | 3 | definition of a nonmetal, not Cl because it is a gas |
35 Based on Reference Table S, atoms of which of these elements have the strongest attraction for the electrons in a chemical bond?
(1) Al (3) P
(2) Si (4) S | 4 | Definition of Electronegativity Use Table S |
36 A sample of a compound contains 65.4 grams of zinc, 12.0 grams of carbon, and 48.0 grams of oxygen. What is the mole ratio of zinc to carbon
to oxygen in this compound?
(1) 1:1:2 (3) 1:4:6
(2) 1:1:3 (4) 5:1:4 | 2 | divide each by the atomic mass Zn 65.4/65.4=1 C 12/12=1 O 48/16=3 |
37 Which process would most effectively separate two liquids with different molecular polarities?
(1) filtration (3) distillation
(2) fermentation (4) conductivity | 3 | molecular polarities= different boiling points |
38 Given the balanced equation:
AgNO3(aq) + NaCl(aq) ==> NaNO3(aq) + AgCl(s)
This reaction is classified as
(1) synthesis
(2) decomposition
(3) single replacement
(4) double replacement | 4 | DR |
39 A solution contains 35 grams of KNO3 dissolved in 100 grams of water at 40°C. How much more KNO3 would have to be added to make it a saturated solution?
(1) 29 g (3) 12 g
(2) 24 g (4) 4 g | 1 | Table G find the difference between 35grams and the line at 40C |
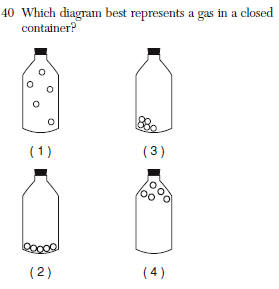 | 1 | that is a gas |
