Questions
(1) melting points of the elements in the compound
(2) densities of the elements in the compound
(3) electronegativities of the bonded atoms in a molecule of the compound
(4) atomic masses of the bonded atoms in a molecule of the compound
(1) It has a definite shape and a definite volume.
(2) It has a definite shape and no definite volume.
(3) It has no definite shape and a definite volume.
(4) It has no definite shape and no definite volume.
(1) C and CuO (3) CO2 and CuO
(2) C and Cu (4) CO2 and Cu
(1) BaSO4 (3) KClO3
(2) CaCrO4 (4) Na2S
heated?
(1) The number of gas molecules increases.
(2) The number of collisions between gas molecules per unit time decreases.
(3) The average velocity of the gas molecules increases.
(4) The volume of the gas decreases.
(1) The catalyst provides an alternate reaction pathway with a higher activation energy.
(2) The catalyst provides an alternate reaction pathway with a lower activation energy.
(3) The catalyst provides the same reaction pathway with a higher activation energy.
(4) The catalyst provides the same reaction pathway with a lower activation energy.
(1) double bonds, only (3) carbon atoms
(2) single bonds, only (4) oxygen atoms
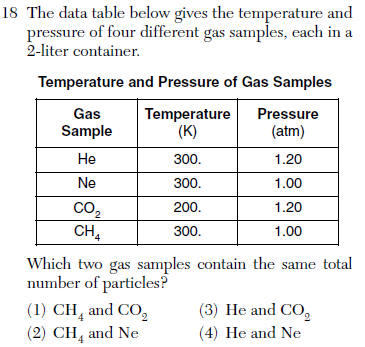
(1) faster and more reactant is produced
(2) faster and more product is produced
(3) the same and the reaction has stopped
(4) the same and the reaction continues in both directions
Concentrations are constant because the rates are equal
(1) ethyne (3) ethanol
(2) ethene (4) ethane