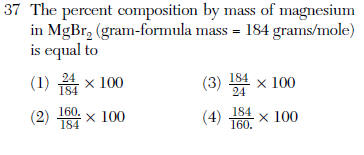Questions | Answer | Explanations |
31 What is the mass number of an atom that has six protons, six electrons, and eight neutrons?
(1) 6 (3) 14
(2) 12 (4) 20 | 3 | 6 protons =atomic # 6 |
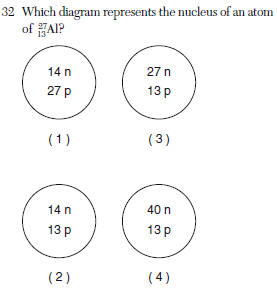 | 2 | mass# of 27= 13p+ and 14no |
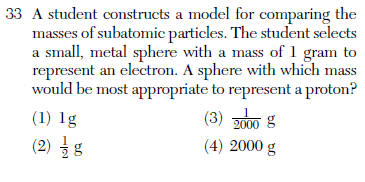 | 4 | an electron mass is approximately 1/1836 of a proton, so the proton is about 2000x more massive |
34 Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond?
(1) metals (3) nonmetals
(2) metalloids (4) noble gases | 3 | fluorine is the greatest, it is a nonmetal |
35 Which list of elements from Group 2 on the Periodic Table is arranged in order of increasing atomic radius?
(1) Be, Mg, Ca (3) Ba, Ra, Sr
(2) Ca, Mg, Be (4) Sr, Ra, Ba | 1 | Table S has the values, put them in order |
36 Which half-reaction shows conservation of charge?
(1) Cu + e- --> Cu+ (3) Cu+ ü--> Cu + e-
(2) Cu2+ + 2e- --> Cu (4) Cu2+ ü-->Cu + 2e- | 2 | The total charge on the left must equal the total charge on the right |
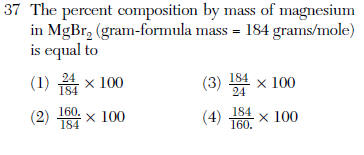 | 1 | plug in mass of MG and divide by total x100% |
38 Given the balanced equation:
CaCO3(s) + 2HCl(aq) ü-->CaCl2(aq) + H2O(l) + CO2(g)
What is the total number of moles of CO2 formed when 20. moles of HCl is completely consumed?
(1) 5.0 mol (3) 20. mol
(2) 10. mol (4) 40. mol | 2 | set up a proportion 20/2 =x/1 |
39 What amount of heat is required to completely melt a 29.95-gram sample of H2O(s) at 0░C?
(1) 334 J (3) 1.00 ū 103 J
(2) 2260 J (4) 1.00 ū 104 J | 4 | melt (fusion) q=mHf= 29.95 x 334 |
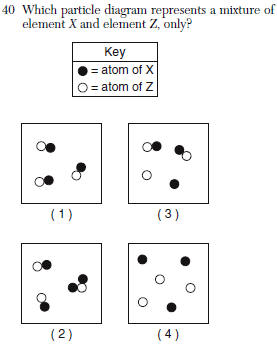 | 4 | elements are not attached |