Questions
(1) protons (3) neutrons
(2) positrons (4) electrons
(1) a positively charged electron cloud surrounding a positively charged nucleus
(2) a positively charged electron cloud surrounding a negatively charged nucleus
(3) a negatively charged electron cloud surrounding a positively charged nucleus
(4) a negatively charged electron cloud surrounding a negatively charged nucleus
cloud negative (electrons)
(1) the mass of 2 electrons
(2) the mass of 2 neutrons
(3) the mass of 1 electron plus the mass of 1 proton
(4) the mass of 1 neutron plus the mass of 1 electron
protons 1 amu
neutrons 1 amu
(1) atomic mass
(2) atomic number
(3) number of neutrons
(4) number of valence electrons
(1) atomic radius
(2) electronegativity
(3) first ionization energy
(4) number of electrons in the first shell
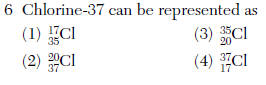
Element
Atomic #
(1) bromine (3) hydrogen
(2) cobalt (4) mercury
(1) CO2, H2O, NH3 (3) H2, Ne, NaCl
(2) H2, N2, O2 (4) MgO, NaCl, O2
(1) FeO (3) Fe3O
(2) Fe2O3 (4) Fe3O=
Fe2O3
(1) chlorine (3) silicon
(2) phosphorus (4) sulfur