Questions
(1) mass (3) temperature
(2) melting point (4) volume
(1) Energy is absorbed as a bond is formed.
(2) Energy is absorbed as a bond is broken.
(3) Energy is released as a bond is formed.
(4) Energy is released as a bond is broken.
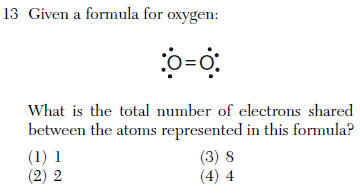
so 4 e-
(1) empirical formulas (3) ion ratios
(2) formula masses (4) physical properties
(1) liters of solute per mole of solution
(2) liters of solution per mole of solution
(3) moles of solute per liter of solution
(4) moles of solution per liter of solution
(1) AlCl3 (3) HI
(2) H2O (4) Cu
(1) compound because the atoms of the elements are combined in a fixed proportion
(2) compound because the atoms of the elements are combined in a proportion that varies
(3) mixture because the atoms of the elements are combined in a fixed proportion
(4) mixture because the atoms of the elements are combined in a proportion that varies
(1) CO2 and HCl (3) H2O and HCl
(2) CO2 and CH4 (4) H2O and CH4
draw them
(1) carbon, only
(2) carbon and hydrogen, only
(3) carbon, hydrogen, and oxygen, only
(4) carbon, hydrogen, oxygen, and nitrogen, only
(1) esterification (3) polymerization
(2) fermentation (4) substitution