Questions | Answer | Explanations |
31 What is the total number of neutrons in the nucleus of a neutral atom that has 19 electrons and a mass number of 39?
(1) 19 (3) 39
(2) 20 (4) 58 | 2 | atom protons =electrons so 19p+ 39-19=20 neutrons |
32 An unknown element X can form a compound with the formula XBr3. In which group on the Periodic Table would element X be found?
(1) 1 (3) 13
(2) 2 (4) 14 | 3 | charge is 3+ group 13 |
33 As the elements in Group 17 on the Periodic Table are considered from top to bottom, what happens to the atomic radius and the metallic character of each successive element?
(1) The atomic radius and the metallic character both increase.
(2) The atomic radius increases and the metallic character decreases.
(3) The atomic radius decreases and the metallic character increases.
(4) The atomic radius and the metallic character both decrease. | 1 | Atomic radius increases Table S Metallic Character increase (bottom left are metals) |
34 Which pair of compounds has the same empirical formula?
(1) C2H2 and C6H6
(2) C2H6 and C3H8
(3) CH3OH and C2H5OH
(4) CH3CHO and CH3COOH | 1 | same ratio |
35 Which equation shows a conservation of mass?
(1) Na + Cl2 --> NaCl
(2) Al + Br2 --> AlBr3
(3) H2O --> H + O2
(4) PCl5 --> PCl3 + Cl2 | 4 | atoms in =atoms out |
36 How many electrons are in an Fe2+ ion?
(1) 24 (3) 28
(2) 26 (4) 56 | 1 | Fe has 26protons and the 2+ means 2 less electrons |
37 A substance that does not conduct electricity as a solid but does conduct electricity when melted is most likely classified as
(1) an ionic compound
(2) a molecular compound
(3) a metal
(4) a nonmetal | 1 | ionic, moving charge =electricity |
38 According to Reference Table H, what is the boiling point of ethanoic acid at 80 kPa?
(1) 28°C (3) 111°C
(2) 100°C (4) 125°C | 3 | match it up |
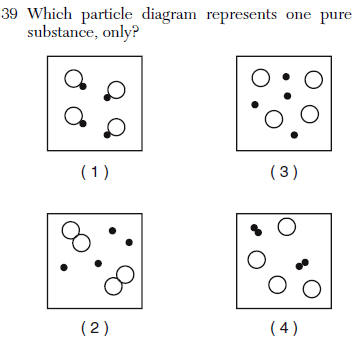 | 1 | only contains the same compound |
40 A sample of helium gas has a volume of 900. milliliters and a pressure of 2.50 atm at 298 K. What is the new pressure when the temperature is changed to 336 K and the volume is decreased to 450. milliliters?
(1) 0.177 atm (3) 5.64 atm
(2) 4.43 atm (4) 14.1 atm | 3 | PV/T =PV/T |
