Questions
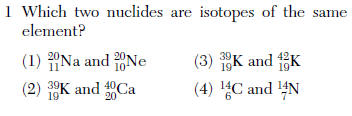
same atomic number (bottom)
(1) emitted by the nucleus
(2) emitted by the electron
(3) absorbed by the nucleus
(4) absorbed by the electron
(1) +13 (3) +5
(2) +9 (4) +4
(1) the atom is a hard sphere =>most of the atom is empty space=> electrons exist in orbitals outside the nucleus
(2) the atom is a hard sphere => electrons exist in orbitals outside the nucleus Å=> most of the atom is empty space
(3) most of the atom is empty space Å=>electrons exist in orbitals outside the nucleus => the atom is a hard sphere
(4) most of the atom is empty space=>the atom is a hard sphere => electrons exist in orbitals outside the nucleus
(1) Oxygen has a melting point of 55 K.
(2) Oxygen can combine with a metal to produce a compound.
(3) Oxygen gas is slightly soluble in water.
(4) Oxygen gas can be compressed.
(1) metal (3) metalloid
(2) noble gas (4) nonmetal
(1) The atomic radius decreases, and the first ionization energy generally increases.
(2) The atomic radius decreases, and the first ionization energy generally decreases.
(3) The atomic radius increases, and the first ionization energy generally increases.
(4) The atomic radius increases, and the first ionization energy generally decreases.
(1) Na2SO3 (3) NaSO3
(2) Na2SO4 (4) NaSO4
2:1 ratio to balance charges
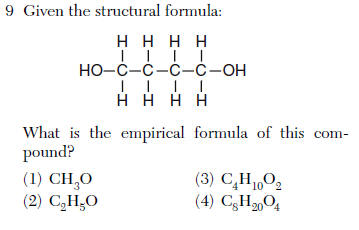
(1) H2(g) + O2(g)=> H2O(g)
(2) N2(g) + H2(g) Å=>NH3(g)
(3) 2NaCl(s)=>Na(s) + Cl2(g)
(4) 2KCl(s) => 2K(s) + Cl2(g)