Questions | Answer | Explanations |
31 Which electron configuration represents the electrons in an atom of chlorine in an excited state?
(1) 2-7-7 (3) 2-8-7
(2) 2-7-8 (4) 2-8-8 | 2 | 17 electrons and one is promoted |
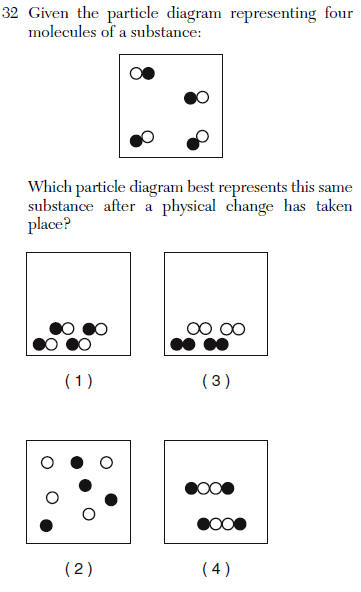 | 1 | physical change is a change in intermolecular distances |
33 What is the percent composition by mass of nitrogen in NH4NO3 (gram-formula mass = 80.0 grams/mole)?
(1) 17.5% (3) 52.5%
(2) 35.0% (4) 60.0% | 2 | (2N x 14 / 80) x 100% |
34 The atomic mass of element A is 63.6 atomic mass units. The only naturally occurring isotopes of element A are A-63 and A-65. The percent abundances in a naturally occurring sample of element A are closest to
(1) 31% A-63 and 69% A-65
(2) 50% A-63 and 50% A-65
(3) 69% A-63 and 31% A-65
(4) 100% A-63 and 0% A-65 | 3 | mostly 63 because the atomic mass is closest to that number. More than 50%. |
35 Elements Q, X, and Z are in the same group on the Periodic Table and are listed in order of increasing atomic number. The melting point of element Q is –219°C and the melting point of element Z is –7°C. Which temperature is closest to the melting point of element X?
(1) –7°C (3) –219°C
(2) –101°C (4) –226°C | 2 | between the -7 and -219 |
36 Given the balanced equation:
2C + 3H2==> C2H6
What is the total number of moles of C that must completely react to produce 2.0 moles of C2H6?
(1) 1.0 mol (3) 3.0 mol
(2) 2.0 mol (4) 4.0 mol | 4 | 2/1 C2H6 = x/ 2C x=4 |
37 Given the balanced equation:
2KClO3==> 2KCl + 3O2
Which type of reaction is represented by this equation?
(1) synthesis
(2) decomposition
(3) single replacement
(4) double replacement | 2 | AB==> A + B decomposition |
38 A solid substance was tested in the laboratory.
The test results are listed below.
• dissolves in water
• is an electrolyte
• melts at a high temperature
Based on these results, the solid substance could be
(1) Cu (3) C
(2) CuBr2 (4) C6H12O6 | 2 | properties of an Ionic compound |
39 If 0.025 gram of Pb(NO3)2 is dissolved in 100. grams of H2O, what is the concentration of the resulting solution, in parts per million?
(1) 2.5 × 10–4 ppm (3) 250 ppm
(2) 2.5 ppm (4) 4.0 × 103 ppm | 3 | Table T ppm, plug in |
40 Given the balanced equation:
4Fe(s) + 3O2(g) ==>2Fe2O3(s) + 1640 kJ
Which phrase best describes this reaction?
(1) endothermic with DH = +1640 kJ
(2) endothermic with DH = -1640 kJ
(3) exothermic with DH = +1640 kJ
(4) exothermic with DH = -1640 kJ | 4 | exothermic, DH = - Table I, on the way bottom |
