Questions
Explanations
31 An ion that consists of 7 protons, 9 neutrons, and 10 electrons has a net charge of
(1) 2- (3) 3+
(2) 2 + (4) 3-
7 +
overall 3-
32 Which electron configuration represents the electrons of an atom in an excited state?
(1) 2–2 (3) 2–8
(2) 2–2–1 (4) 2–8–1
2-2-1 is excited
look at element number 5 for the ground state config
33 The table below gives the atomic mass and the abundance of the two naturally occurring isotopes of boron.

Which numerical setup can be used to determine the atomic mass of the element boron?
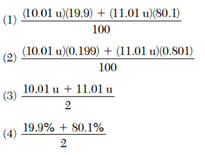
or
(mass x percent) +(mass x percent)
---------------------------------------
100%
34 In which group on the Periodic Table would a nonmetallic element belong if atoms of this element tend to gain two electrons to complete their valence shell?
(1) 14 (3) 16
(2) 15 (4) 17
gain 2 more to get their octet
35 Which trend is observed as the first four elements in Group 17 on the Periodic Table are considered in order of increasing atomic number?
(1) Electronegativity increases.
(2) First ionization energy decreases.
(3) The number of valence electrons increases.
(4) The number of electron shells decreases.
bottom has lower
use table S