Questions
Explanations
36 What is the number of moles of KF in a 29-gram sample of the compound?
(1) 1.0 mol (3) 0.50 mol
(2) 2.0 mol (4) 5.0 mol
37 Which bond is most polar?
(1) C–O (3) N–O
(2) H–O (4) S–O
table S electronegativity
38 Based on Table F, which equation represents a saturated solution having the lowest concentration of Cl- ions?
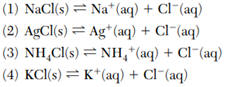
39 What is the molarity of a solution that contains 0.500 mole of KNO3 dissolved in 0.500-liter of solution?
(1) 1.00 M (3) 0.500 M
(2) 2.00 M (4) 4.00 M
40 Given samples of water:
Sample 1: 100. grams of water at 10.°C
Sample 2: 100. grams of water at 20.°C
Compared to sample 1, sample 2 contains
(1) molecules with a lower average kinetic energy
(2) molecules with a lower average velocity
(3) less heat energy
(4) more heat energy