Questions
Explanations
41 Which particle diagram represents one substance in the gas phase?
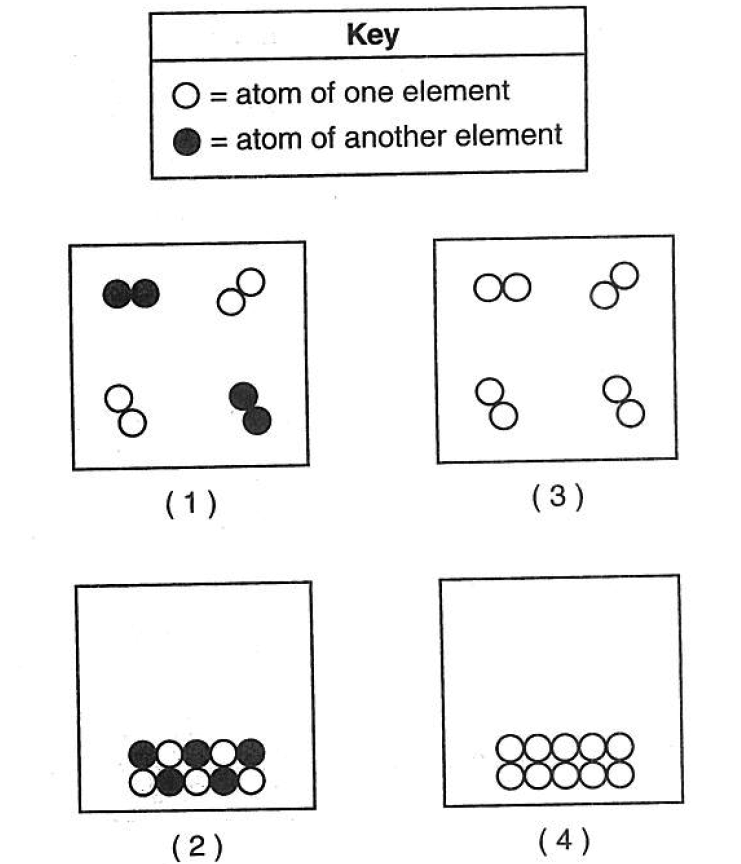
they must be the same compounds or elements to be one substance
42 Given the equation representing a chemical reaction at equilibrium in a sealed, rigid container:
H2(g) + I2(g) + energy --> 2HI(g)
When the concentration of H2(g) is increased by adding more hydrogen gas to the container at constant temperature, the equilibrium shifts
(1) to the right, and the concentration of HI(g) decreases
(2) to the right, and the concentration of HI(g) increases
(3) to the left, and the concentration of HI(g) decreases
(4) to the left, and the concentration of HI(g) increases
LeChaterliers Principle
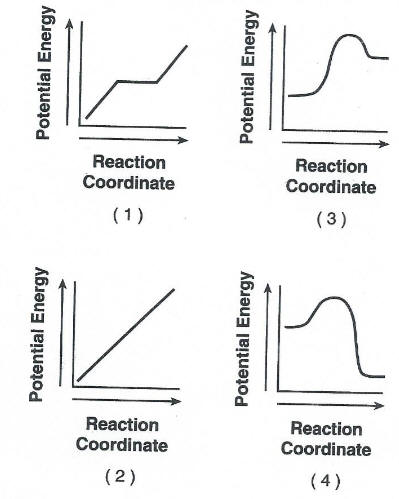
releasing energy
(1) CH3CHO
(2) CH3OCH3
(3) CH3COCH3
(4) CH3COOCH3
probably should expand them and make sure carbon has 4 bonds to spot the ether
3 is a ketone
45 Given the equation representing a reversible reaction:
HCO- (aq) + H2O(l) <--> H2CO3 (aq) + OH-(aq)
Which formula represents the H+ acceptor in the forward reaction?(1) HCO- (aq)
(2) H2O(l)
(3) H2CO3 (aq)
(4) OH-(aq)
HCO- ==> H2CO3