Questions
Explanations
46 What is the mass of an original 5.60-gram sample of iron-53 that remains unchanged after 25.53 minutes?
(1) 0.35g (3) 1.40 g
(2) 0.70 g (4) 2.80 g
25.53 min/8.51 min= 3 half lives
5.60g-->2.80g-->1.40g-->0.70g
47 Given the equation representing a nuclear reaction:
| 1 | H + X | ==> | 6 | Li | + | 4 | He |
| 1 | 3 | 2 |
| (1)
|
| (3) |
| ||||||
| (2) |
| (4) |
|
mass in equals mass out
atomic numbers in equal atomic numbers out
atomic number determines the element
(1) require elements with large atomic numbers
(2) create radioactive products
(3) use radioactive reactants
(4) combine light nuclei
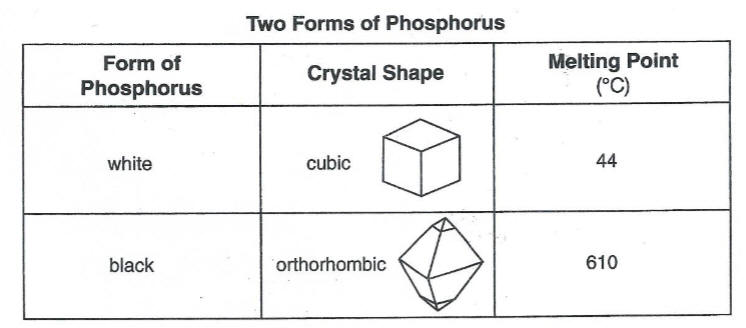
Which phrase describes the two forms of phosphorus?
(l) same crystal structure and same properties
(2) same crystal structure and different properties
(3) different crystal structures and different properties
(4) different crystal structures and same properties
allotropes same element different forms, different properties