|
Matter Terminology Classifying Matter Phases of Matter Physical and Chemical Changes Separation Techniques Vapor Pressure Phase Changes Heating Curve Phase Diagrams | | Endothermic and Exothermic Processes | |

Exothermic- the word describes a process that releases energy in the form of heat. Forming a chemical bond releases energy and therefore is an exothermic process. Exothermic reactions usually feel hot because it is giving heat to you. Endothermic - a process or reaction that absorbs energy in the form of heat. Breaking a chemical bond requires energy and therefore is Endothermic. Endothermic reactions usually feel cold because it is taking heat away from you. 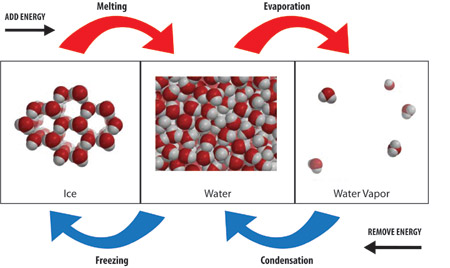
| Exothermic Processes | Endothermic Processes | freezing water solidifying solid salts condensing water vapor making a hydrate from an anhydrous salt forming an anion from an atom in the gas phase Annihilation of matter E=mc2 splitting of an atom
| | | Exothermic Reactions | Endothermic Reactions | Combustion of hydrogen dissolving lithium chloride in water Burning of propane dehydration of sugar with sulfuring acid thermite decomposition of hydrogen peroxide decomposition od ammonium dichromate halogenation of acetylene
| Reaction of barium hydroxide octahydrate crystals with dry ammonium chloride dissolving ammonium chloride in water reaction of thionyl chloride (SOCl2) with cobalt(II) sulfate heptahydrate mixing water and ammonium nitrate mixing water with potassium chloride reacting ethanoic acid with sodium carbonate photosynthesis (chlorophyll is used to react carbon dioxide plus water plus energy to make glucose and oxygen)
|
Matter Terminology Classifying Matter Phases of Matter Physical and Chemical Changes Separation Techniques Vapor Pressure Phase Changes Heating Curve Phase Diagrams  Chemical Demonstration Videos Chemical Demonstration Videos
| 
