Questions | Answer | Explanations |
| 1 Subatomic particles can usually pass undeflected through an atom because the volume of an atom is composed of (1) an uncharged nucleus (2) largely empty space (3) neutrons (4) protons | 2 | Atoms are mostly open space, it went through. |
| 2 What is the total number of electrons in the valence shell of an atom of aluminum in the ground state? (1) 8 (3) 3 (2) 2 (4) 10 | 3 | last number of electrons 2-8-3 |
| 3 Which of these elements has physical and chemical properties most similar to silicon (Si)? (1) germanium (Ge) (3) phosphorus (P) (2) lead (Pb) (4) chlorine (Cl) | 1 | Same Group |
| 4 What is the total number of protons in the nucleus of an atom of potassium-42? (1) 15 (3) 39 (2) 19 (4) 42 | 2 | Atomic number of potassium |
| 5 Given the equation: H2O(s) <-->H2O(l) At which temperature will equilibrium exist when the atmospheric pressure is 1 atm? (1) 0 K (3) 273 K (2) 100 K (4) 373 K | 3 | melting point of water (also freezing pt) |
| 6 Which species represents a chemical compound? (1) N2 (3) Na (2) NH4+ (4) NaHCO3 | 4 | 2 or more bonded element (neutral) |
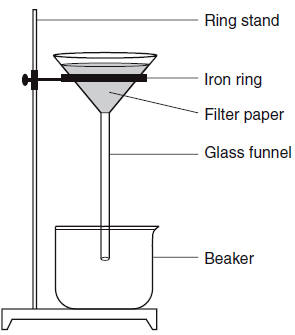
| 7 Which mixture can be separated by using the equipment shown below?
| 1 | filtrations separates out solids from liquids and aq |
| 8 Which reaction represents natural nuclear decay? 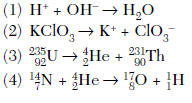 | 3 | natural 1 reactant, also look for the isotopic notation |
| 9 If an equation is balanced properly, both sides of the equation must have the same number of (1) atoms (2) coefficients (3) molecules (4) moles of molecules | 1 | atoms |
| 10 Which of the following elements has the highest electronegativity? (1) H (3) Al (2) K (4) Ca | 1 | tableS |