Questions | Answer | Explanations |
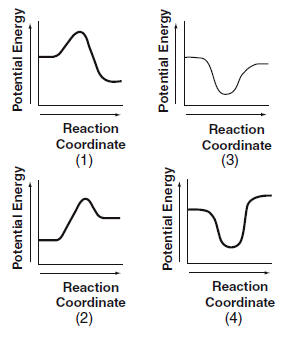
| 41 According to Table I, which potential energy diagram best represents the reaction that forms H2O(l) from its elements?
| 1 | DH is negative, exothermic |
42 Which structural formula is incorrect?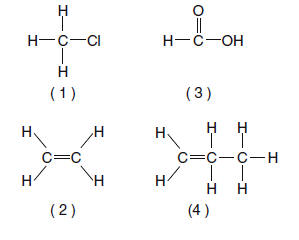 | 4 | central carbon is bonded 5 times |
 | 4 | add the top number and bottom numbers |
44 The vapor pressure of a liquid is 0.92 atm at 60°C. The normal boiling point of the liquid could be (1) 35°C (3) 55°C (2) 45°C (4) 65°C | 4 | since it is a liquid at 60°C it must boil at a higher temp |
| 45 When 50. milliliters of an HNO3 solution is exactly neutralized by 150 milliliters of a 0.50 M solution of KOH, what is the concentration of HNO3? (1) 1.0 M (3) 3.0 M (2) 1.5 M (4) 0.5 M | 2 | MV=MV (150mL x 0.50M)/50mL = |
| 46 In Period 3, from left to right in order, each successive element will (1) decrease in electronegativity (2) decrease in atomic mass (3) increase in number of protons (4) increase in metallic character | 3 | Table S |
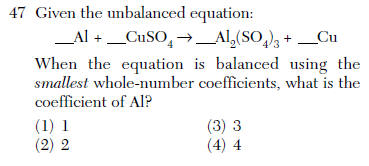 | 2 | 2 balance it |
| 48 One hundred grams of water is saturated with NH4Cl at 50°C. According to Table G, if the temperature is lowered to 10°C, what is the total amount of NH4Cl that will precipitate? (1) 5.0 g (3) 30. g (2) 17 g (4) 50. g | 2 | subtract the amount at 50C from the amount at 10C 52-34= 18grams close enough |
| 49 What is the total number of grams of NaI(s) needed to make 1.0 liter of a 0.010 M solution? (1) 0.015 (3) 1.5 (2) 0.15 (4) 15 | 3 | 2 parter M=mol/L mol=grams /gram formula mass |
| 50 Given the reaction: 2 H2(g) + O2(g)-->2 H2O(l) + 571.6 kJ What is the approximate DH for the formation of 1 mole of H2O(l)? (1) .285.8 kJ (3) .571.6 kJ (2) +285.8 kJ (4) +571.6 kJ | 1 | DH is negative exothermic for 1 mole 285.6kJ is released that reaction is for 2 moles of water |