Questions | Answer | Explanations |
| 31 Compared to the nonmetals in Period 2, the metals in Period 2 generally have larger (1) ionization energies (2) electronegativities (3) atomic radii (4) atomic numbers | 3 | Table S, look them up |
| 32 Which of the following Group 2 elements has the lowest first ionization energy? (1) Be (3) Ca (2) Mg (4) Ba | 4 | Table S, look them up |
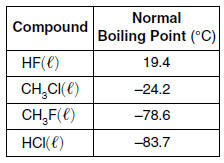
| 33 The table below shows the normal boiling point of four compounds.
| 1 | Strongest IMF, highest BP, also H-bonding |
| 34 According to Table I, which salt releases energy as it dissolves? (1) KNO3 (3) NH4NO3 (2) LiBr (4) NaCl | 2 | exo, DH is negative |
| 35 During a laboratory activity, a student combined two solutions. In the laboratory report, the student wrote ďA yellow color appeared.Ē The statement represents the studentís recorded (1) conclusion (3) hypothesis (2) observation (4) inference | 2 | observation, I refuse to explain this |
| 36 How many moles of solute are contained in 200 milliliters of a 1 M solution? (1) 1 (3) 0.8 (2) 0.2 (4) 200 | 2 | M=mol/L 200mL=0.200L |
| 37 Increasing the temperature increases the rate of a reaction by (1) lowering the activation energy (2) increasing the activation energy (3) lowering the frequency of effective collisions between reacting molecules (4) increasing the frequency of effective collisions between reacting molecules | 4 | reactions= effective collisions |
| 38 Given the equilibrium reaction in a closed system: H2(g) + I2(g) + heat-->2 HI(g) What will be the result of an increase in temperature? (1) The equilibrium will shift to the left and [H2] will increase. (2) The equilibrium will shift to the left and [H2] will decrease. (3) The equilibrium will shift to the right and [HI] will increase. (4) The equilibrium will shift to the right and [HI] will decrease. | 3 | shifts right, HI will increase |
| 39 Which sample has the lowest entropy? (1) 1 mole of KNO3(l) (3) 1 mole of H2O(l) (2) 1 mole of KNO3(s) (4) 1 mole of H2O(g) | 2 | Solids lowest gases highest |
| 40 Which of the following compounds is least soluble in water? (1) copper (II) chloride (2) aluminum acetate (3) iron (III) hydroxide (4) potassium sulfate | 3 | Table F, Insoluble=least soluble |