| Questions | Answers | Explanation |
| 21. Which type of reaction occurs when nonmetal atoms become negative nonmetal ions? A. oxidation
B. reduction
C. substitution
D. condensation | B | reduction the oxidation number (charge) becomes negative, it decreases or reduces |
22. Given the reaction:
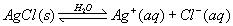 Once equilibrium is reached, which statement is accurate? A. The concentration of Ag+(aq) is greater than the concentration of Cl–(aq).
B. The AgCl(s) will be completely consumed.
C. The rates of the forward and reverse reactions are equal.
D. The entropy of the forward reaction will continue to decrease.
| C | ALWAYS ON REGENTS equilibrium rates are equal concentrations constant |
| 23. Which structural formula correctly represents a hydrocarbon molecule? A.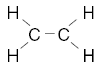
B.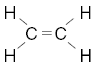
C.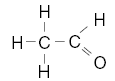
D.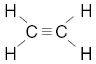 | B | carbon must have 4 bonds H has 1 O has 2 |
24. Given the structural formulas for two organic compounds:
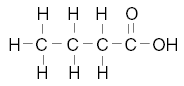 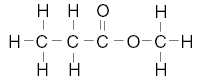 The differences in their physical and chemical properties are primarily due to their
different A. number of carbon atoms
B. number of hydrogen atoms
C. molecular masses
D. functional groups | D | the functional groups are different |
| 25. Which structural formula represents a molecule that is not an isomer of pentane? A.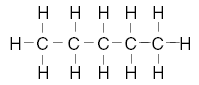
B.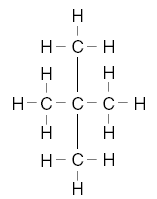
C.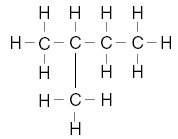
D.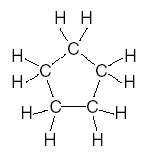 | D | isomers same formula different structure Note A is pentane, not an isomer of |
| 26. The bonds in the compound MgSO4 can be described as A. ionic, only
B. covalent, only
C. both ionic and covalent
D. neither ionic nor covalent | C | SO4 2- (sulfate is covalent) MgSO4 is ionic so there is both |
| 27. Given the reaction: 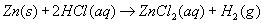
Which statement correctly describes what occurs when this reaction takes place in a closed system? A. Atoms of Zn(s) lose electrons and are oxidized.
B. Atoms of Zn(s) gain electrons and are reduced.
C. There is a net loss of mass.
D. There is a net gain of mass. | A | the Zn becomes Zn+2 charge went up...oxidation it is oxidized by losing electrons LEO GER |
| 28. When the pH of a solution changes from a pH of 5 to a pH of 3, the hydronium ion concentration is A. 0.01 of the original content
B. 0.1 of the original content
C. 10 times the original content
D. 100 times the original content | D | each pH unit change is 10 times more concentrated in H3O+ 5 to 3 is 100 times more H3O+ |
| 29. A sample of Ca(OH)2 is considered to be an Arrhenius base because it dissolves in water to yield A. Ca2+ ions as the only positive ions in solution
B. H3O+ ions as the only positive ions in solution
C. OH– ions as the only negative ions in solution
D. H– ions as the only negative ions in solution | C | Base hydroxide ions acids hydronium or hydrogen ions |
| 30. Which reaction occurs when hydrogen ions react with hydroxide ions to form water? A. substitution
B. saponification
C. ionization
D. neutralization
| D | hydogen and hydroxide making water neutralization |