| Questions | Answers | Explanantions |
| 41. Which equation represents a double replacement reaction? A.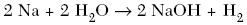
B.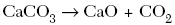
C.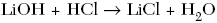
D.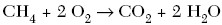
| C | positive ions switch with the negative ions |
| 42. What is the molecular formula of a compound that has a molecular mass of 54 and the empirical formula C2H3? A. C2H3
B. C4H6
C. C6H9
D. C8H12 | B | C4H6 has a mass of 54 and can be reduced to C2H3 |
| 43. Given the diagrams X, Y, and Z below: 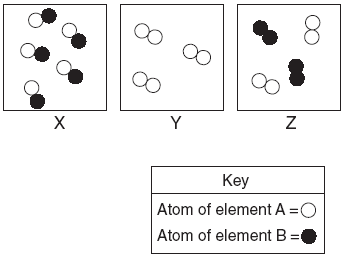
Which diagram or diagrams represent a mixture of elements A and B? A. X, only
B. Z, only
C. X and Y
D. X and Z | B | X is a compound Y is a diatomic element Z is 2 diatomic elements |
| 44. Which is an electron configuration for an atom of chlorine in the excited state? A. 2–8–7
B. 2–8–8
C. 2–8–6–1
D. 2–8–7–1 | C | 17 electrons with 1 promoted to a higher level |
| 45. Based on the nature of the reactants in each of the equations below, which reaction at 25°C will occur at the fastest rate? A.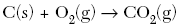
B.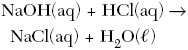
C.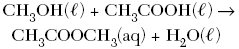
D.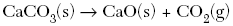
| B | ionic substances dissolved react the fastest |
| 46. Given the reaction at equilibrium: 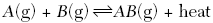
The concentration of A(g) can be increased by A. lowering the temperature
B. adding a catalyst
C. increasing the concentration of AB(g)
D. increasing the concentration of B(g)
| C | LeChatelier- adding to one side shifts the equilibrium to the other taking away will bring it back temp is heat |
| 47. Which structural formula represents an alcohol? A.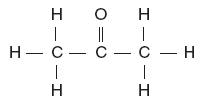
B.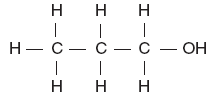
C.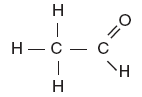
D.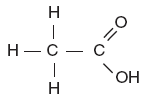 | B | -OH alcohol |
| 48. A voltaic cell differs from an electrolytic cell in that in a voltaic cell A. energy is produced when the reaction occurs
B. energy is required for the reaction to occur
C. both oxidation and reduction occur
D. neither oxidation nor reduction occurs | A | voltaic is a battery electrolytic requires one |
| 49. What is the purpose of the salt bridge in a voltaic cell? A. It blocks the flow of electrons.
B. It blocks the flow of positive and negative ions.
C. It is a path for the flow of electrons.
D. It is a path for the flow of positive and negative ions. | D | ion flow (salt--- ions) |
| 50. According to Reference Table N, which radioactive isotope will retain only 1/8 its original radioactive atoms after approximately 43 days? A. gold-198
B. iodine-131
C. phosphorus-32
D. radon-222 | C | 1/8= 3 (1/2 lives) 43days/3= 14.3d P-32 |