| Questions | Answer | Explanations |
| 31. Which of these types of nuclear radiation has the greatest penetrating power? A. alpha
B. beta
C. neutron
D. gamma | D | gamma strongest beta than alpha |
| 32.Alpha particles and beta particles differ in A. mass, only
B. charge, only
C. both mass and charge
D. neither mass nor charge | C | alpha helium nucleus (+) mass 4 beta electron (-) mass almost 0 |
33. Given the nuclear reaction:
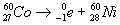 This reaction is an example of A. fission
B. fusion
C. artificial transmutation
D. natural transmutation | D | naturat transmutation 1 reactant artifical transmutation 2 reactants element change is transmutation |
| 34. As two chlorine atoms combine to form a molecule, energy is A. absorbed
B. released
C. created
D destroyed | B | making a bond exo breaking endo |
| 35. In most aqueous reactions as temperature increases, the effectiveness of collisions between reacting particles A. decreases
B. increases
C.remains the same | B | higher temperatures faster molecules more effective collisions |
| 36. What is the total number of neutrons in an atom of an element that has a mass number of 19 and an atomic number of 9? A. 9
B. 10
C. 19
D. 28
| B | neutrons=mass number (p +n) - atomic number (p) |
| 37. The element in Period 4 and Group 1 of the Periodic Table would be classified as a A. metal
B. metalloid
C. nonmetal
D. noble gas | A | potassium is a metal |
| 38. As the elements in Period 2 of the Periodic Table are considered in succession from left to right, there is a decrease in atomic radius with increasing atomic number. This may best be explained by the fact that the A. number of protons increases, and the number of shells of electrons remains the same
B. number of protons increases, and the number of shells of electrons increases
C. number of protons decreases, and the number of shells of electrons remains the same
D. number of protons decreases, and the number of shells of electrons increases | A | the number of shells remains constant and the protons increase in number. This pulls the electrons closer to the nucleus. |
| 39. Given the balanced equation: 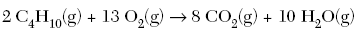
What is the total number of moles of O2(g) that must react completely with 5.00 moles of C4H10(g)? A. 10.0
B. 20.0
C. 26.5
D. 32.5 | D | set up a proportion using the balanced equation 5 moles C4H10(g) is to 2 C4H10 as X moles of O2 is to 13 O2 5/2 = X/13 |
| 40. Which particle has the same electron configuration as a potassium ion? A. fluoride ion
B. sodium ion
C. neon atom
D. argon atom | D | a potassium ion has 18 electrons just like argon |