Questions
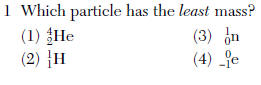
see the zero in #4
(1) the atomic mass of each artificially produced isotope of chlorine, only
(2) the relative abundance of each naturally occurring isotope of chlorine, only
(3) the atomic mass and the relative abundance of each naturally occurring isotope of chlorine
(4) the atomic mass and the relative abundance of each naturally occurring and artificially produced isotope of chlorine
(1) equals the number of electrons
(2) equals the number of neutrons
(3) is less than the number of electrons
(4) is greater than the number of electrons
(1) absorbing energy (3) gaining an electron
(2) releasing energy (4) losing an electron
(1) They have different molecular structures, only.
(2) They have different properties, only.
(3) They have different molecular structures and different properties.
(4) They have the same molecular structure and the same properties.
(1) Bromine is soluble in water.
(2) Bromine has a reddish-brown color.
(3) Bromine combines with aluminum to produce AlBr3.
(4) Bromine changes from a liquid to a gas at 332 K and 1 atm.
physical are phase changes (including dissolving)
(1) mass
(2) electronegativity
(3) total number of protons
(4) total number of valence electrons
(1) solution
(2) compound
(3) homogeneous mixture
(4) heterogeneous mixture
(1) ionic compound
(2) molecular compound
(3) metallic element
(4) nonmetallic element
(1) The shape of the CO2 molecule is symmetrical.
(2) The shape of the CO2 molecule is asymmetrical.
(3) The CO2 molecule has a deficiency of electrons.
(4) The CO2 molecule has an excess of electrons.
Symmertrical is Nonpolar, Asymmertical is Polar