Questions | Answer | Explanations |
31 Which electron configuration represents an
excited state for a potassium atom?
(1) 2-8-7-1 (3) 2-8-8-1
(2) 2-8-7-2 (4) 2-8-8-2 | 2 | 19 electrons and 1 is promoted |
32 A sample of an element is malleable and can conduct electricity. This element could be
(1) H (3) S
(2) He (4) Sn | 4 | properties of metals |
33 Which general trend is demonstrated by the Group 17 elements as they are considered in order from top to bottom on the Periodic Table?
(1) a decrease in atomic radius
(2) a decrease in electronegativity
(3) an increase in first ionization energy
(4) an increase in nonmetallic behavior | 2 | Table S |
34 Which element is a liquid at 758 K and standard pressure?
(1) gold (3) platinum
(2) silver (4) thallium | 4 | Table S Melting Points |
35 Which equation represents a decomposition reaction?
(1) CaCO3(s) ==>CaO(s) + CO2(g)
(2) Cu(s) + 2AgNO3(aq) ==>2Ag(s) + Cu(NO3)2(aq)
(3) 2H2(g) + O2(g) ==>2H2O(l)
(4) KOH(aq) + HCl(aq) ==>KCl(aq) + H2O(l) | 1 | decomp AB==> A + B |
36 A compound has the empirical formula CH2O and a gram-formula mass of 60. grams per mole. What is the molecular formula of this compound?
(1) CH2O (3) C3H8O
(2) C2H4O2 (4) C4H8O4 | 2 | CH2O= 30g/mol C2H4O2 =60g/mol |
37 Which formula represents strontium phosphate?
(1) SrPO4 (3) Sr2(PO4)3
(2) Sr3PO8 (4) Sr3(PO4)2 | 4 | Sr2+ PO43- Sr3(PO4)2 |
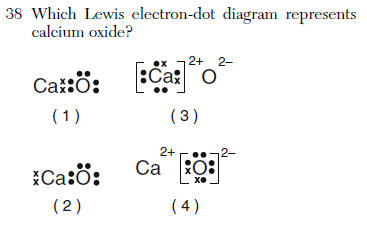 | 4 | Ionic Metals no dots + charge Nonmetals 8 dots - charge |
39 Which statement describes the transfer of heat energy that occurs when an ice cube is added to an insulated container with 100 milliliters of
water at 25°C?
(1) Both the ice cube and the water lose heat energy.
(2) Both the ice cube and the water gain heat energy.
(3) The ice cube gains heat energy and the water loses heat energy.
(4) The ice cube loses heat energy and the water gains heat energy. | 3 | heat goes from HOT TO COLD |
40 What is the mass of NH4Cl that must dissolve in 200. grams of water at 50.°C to make a saturated solution?
(1) 26 g (3) 84 g
(2) 42 g (4) 104 g | 4 | Table G...DOUBLE the results it is 200g of Water |
