Questions | Answer | Explanations |
1 Which subatomic particles are located in the nucleus of a neon atom?
(1) electrons and positrons
(2) electrons and neutrons
(3) protons and neutrons
(4) protons and electrons | 3 | nucleus= protons and neutrons |
2 The total mass of the protons in an atom of gold-198 is approximately
(1) 79 atomic mass units
(2) 119 atomic mass units
(3) 198 atomic mass units
(4) 277 atomic mass units | 1 | atomic number of gold is 79= #protons each proton is 1 amu |
3 In a calcium atom in the ground state, the electrons that possess the least amount of energy are located in the
(1) first electron shell
(2) second electron shell
(3) third electron shell
(4) fourth electron shell | 1 | least energy lowest energy level, so 1 |
4 Which group of atomic models is listed in historical order from the earliest to the most recent?
(1) hard-sphere model, wave-mechanical model, electron-shell model
(2) hard-sphere model, electron-shell model, wave-mechanical model
(3) electron-shell model, wave-mechanical model, hard-sphere model
(4) electron-shell model, hard-sphere model, wave-mechanical model | 2 | Dalton, Bohr, Wave Model |
 | 4 | that is it Table N |
6 An atom of argon rarely bonds to an atom of another element because an argon atom has
(1) 8 valence electrons
(2) 2 electrons in the first shell
(3) 3 electron shells
(4) 22 neutrons | 1 | filled valence shell of 8 e- |
7 The elements on the Periodic Table are arranged in order of increasing
(1) boiling point (3) atomic number
(2) electronegativity (4) atomic mass | 3 | atomic number, look at it |
8 Which element is classified as a nonmetal?
(1) Be (3) Si
(2) Al (4) Cl | 4 | right side of the staircase |
9 Solid samples of the element phosphorus can be white, black, or red in color. The variations in color are due to different
(1) atomic masses
(2) molecular structures
(3) ionization energies
(4) nuclear charges | 2 | different molecular structures have different properties |
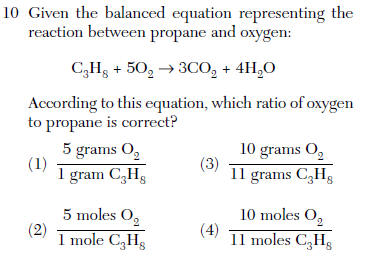 | 2 | balanced reaction are mole ratios |

