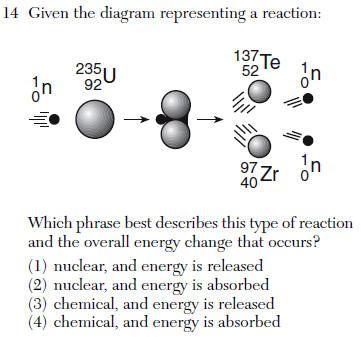
Questions
(1) mass number
(2) atomic number
(3) number of electron shells
(4) number of valence electrons
(1) C6H12O6(s) and KI(s)
(2) C6H12O(s) and HCl(g)
(3) KI(s) and NaCl(s)
(4) NaCl(s) and HCl(g)
NO METALS
(1) C2H5OH (3) C12H22O11
(2) C6H12O6 (4) CH3COOH
(1) more electrons and a larger radius
(2) more electrons and a smaller radius
(3) fewer electrons and a larger radius
(4) fewer electrons and a smaller radius
Br2-->Br + Br
What occurs during this change?
(1) Energy is absorbed and a bond is formed.
(2) Energy is absorbed and a bond is broken.
(3) Energy is released and a bond is formed.
(4) Energy is released and a bond is broken.
(1) tungsten (3) krypton
(2) antimony (4) methane
(1) a compound (3) a mixture
(2) an element (4) a substance
(1) lower boiling point and a higher freezing point
(2) lower boiling point and a lower freezing point
(3) higher boiling point and a higher freezing point
(4) higher boiling point and a lower freezing point
the higher the BP, and lower the FP
(1) 2Ca(s) + O2(g) ü=>2CaO(s)
(2) AgNO3(aq) + KCl(aq) =>AgCl(s) + KNO3(aq)
(3) HCl(aq) + NaOH(aq) ü=>NaCl(aq) + H2O(l)
(4) H3O+(aq) + OH-(aq) ü=> 2H=O(l)
look for a free element (not Ion)