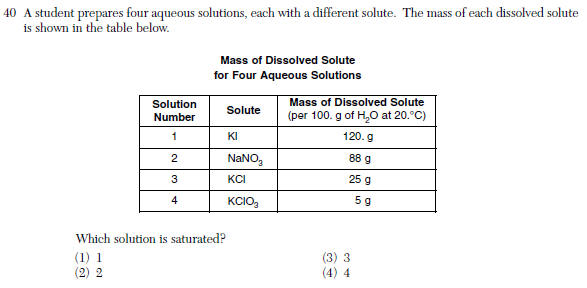
Questions
(1) a higher melting point and a higher boiling point
(2) a higher melting point and a lower boiling point
(3) a lower melting point and a higher boiling point
(4) a lower melting point and a lower boiling point
(1) 24Mg and 12 protons
(2) 28Si and 14 protons
(3) 75As and 75 protons
(4) 80Br and 80 protons
atomic # is the number of protons
(1) Hf, Hg, He (3) Ba, Br2, B
(2) Cr, Cl2, C (4) Se, Sn, Sr
He and Cl2 are gases
the rest are solids
To which group on the Periodic Table does element X belong?
(1) Group 8 (3) Group 13
(2) Group 2 (4) Group 16
so group 16
(1) 154.3 g (3) 171.3 g
(2) 155.3 g (4) 308.6 g
4NH3 + 5O2 ==>4NO + 6H2O
What is the minimum number of moles of O2 that are needed to completely react with 16 moles of NH3?
(1) 16 mol (3) 64 mol
(2) 20. mol (4) 80. mol
16/4 = x/5
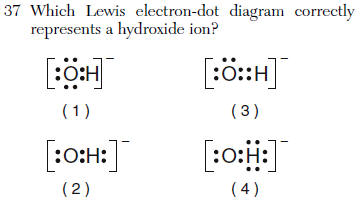
oxygen gets an octet H gets a duet of electrons
(1) aluminum (3) titanium
(2) magnesium (4) zinc
(1) 25°C (3) 273 K
(2) 37°C (4) 298 K
37C is 310K