Questions
Explanations
(1) C2H4 (2) C3H8
(3) С4Н10 (4) С5Н12
not CnH2n+2
(1) I-131 (2) U-238
(3) Ca-37 (4) Fr-220
just memorize it
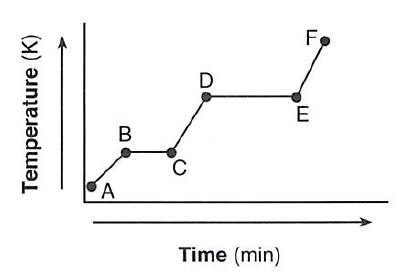
Which statement describes the energy of the particles in this sample during interval DE?
(1) Both potential energy and average kinetic energy increase.
(2) Both potential energy and average kinetic energy decrease.
(3) Potential energy increases and average kinetic energy remains the same.
(4) Potential energy remains the same and average kinetic energy increases.
Potential energy increases and average kinetic energy remains the same
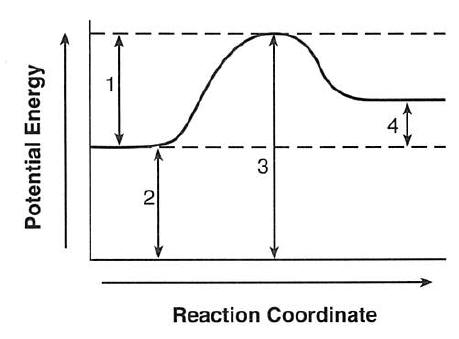
Which intervals are affected by the addition of a catalyst?
(1) 1 and 2 (2) 1 and 3
(3) 2 and 4 (4) 3and 4
(1) Mg + Cl2 ==> MgCl2
(2) CaO + H2O ==> Ca(OH)2
(3) HNO3 + NaOH ==> NaNO3 + H2O
(4) NaCl + AgNO3 ==>AgCl + NaNO3