Questions | Answer | Explanations |
11 Which of the following atoms has the greatest tendency to attract electrons?
| 4 | Definition of Electronegativity Table J KNOW IT |
12 Which 5.0-milliliter sample of NH3 will take the shape of and completely fill a closed 100.0-milliliter container?
| 3 | Gases complete fill a closed container. |
13 The strongest forces of attraction occur between molecules of
| 2 | Forces of Attraction + Hydrogen Compounds= H-Bonding Questions HF, NH3, H2O |
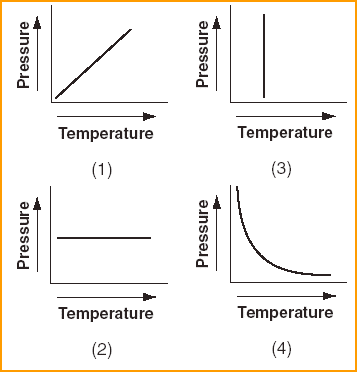
| 14 Which graph shows the pressure-temperature relationship expected for an ideal gas?
| 1 | Direct Relationship P T V |
| 15 At the same temperature and pressure, which sample contains the same number of moles of particles as 1 liter of O2(g)? (1) 1 L Ne(g) (3) 0.5 L SO2(g)
| 1 | Avagodros Hypothesis Different Gases, at the same conditions Same volumes; Same # of Molecules |
| 16 Which change in the temperature of a 1-gram sample of water would cause the greatest increase in the average kinetic energy of its molecules?
| 3 | Definition of Temperature (Average Kinetic Energy) |
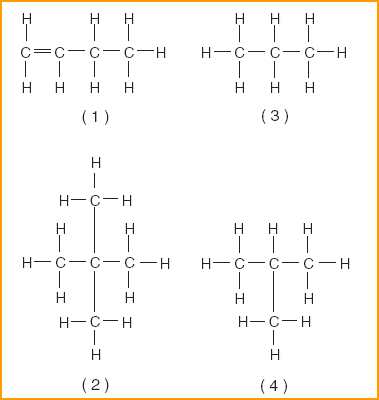
17 Which compound is classified as a hydrocarbon?
| 1 | Hydrocarbon- Hydrogen and carbon only |
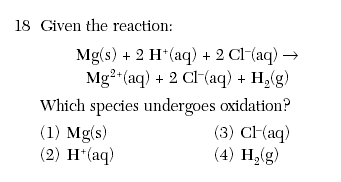
| 1 | Oxidation the ox. Number increases Don't even worry about electrons in this question. |
| 19 Which formula is an isomer of butane? | 4 | Definition-Isomer, same formula different structure Butane-4 carbons all single bonded to hydrogen also CnH2n+2 n=4 C4H10 |
20 Which particles are gained and lost during a redox reaction?
| 1 | Redox-electrons transfer, oxidation numbers change |