Questions | Answer | Explanations |
| 31 In which shell are the valence electrons of the elements in Period 2 found? (1) 1 (3) 3 (2) 2 (4) 4 | 2 | period number is the number of principle energy levels |
| 32 Which of the following Group 15 elements has the greatest metallic character? (1) nitrogen (3) antimony (2) phosphorus (4) bismuth | 4 | the one at the bottom |
| 33 The number of neutrons in the nucleus of an atom can be determined by (1) adding the atomic number to the mass number (2) subtracting the atomic number from the mass number (3) adding the mass number to the atomic mass (4) subtracting the mass number from the atomic number | 2 | mass #- atomic # |
| 34 A compound has a gram formula mass of 56 grams per mole. What is the molecular formula for this compound? (1) CH2 (3) C3H6 (2) C2H4 (4) C4H8 | 4 | use PT to find the mass |
| 35 Given the equilibrium reaction at STP: N2O4(g) <==> 2 NO2(g) Which statement correctly describes this system? (1) The forward and reverse reaction rates are equal. (2) The forward and reverse reaction rates are both increasing. (3) The concentrations of N2O4 and NO2 are equal. (4) The concentrations of N2O4 and NO2 are both increasing. | 1 | concentrations are constant and rates are equal |
| 36 What is the total number of oxygen atoms in the formula MgSO4•7H2O? [The • represents seven units of H2O attached to one unit of MgSO4.] (1) 11 (3) 5 (2) 7 (4) 4 | 1 | 4 from MgSO4 7 from 7 waters |
| 37 Given the reaction: 6 CO2 + 6 H2O==> C6H12O6 + 6 O2 What is the total number of moles of water needed to make 2.5 moles of C6H12O6? (1) 2.5 (3) 12 (2) 6.0 (4) 15 | 4 | proportion 2.5/1C6H12O6 = X/6 H2O |
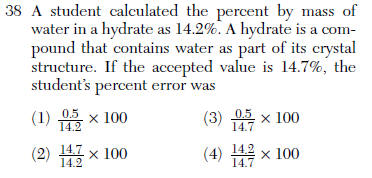 | 3 | table T |
| 39 Which of the following ions has the smallest radius? (1) F– (3) K+ (2) Cl– (4) Ca2+ | 4 | Ca2+ lost 2 electrons F– if all the ions were +1 or -1 |
| 40 According to Reference Table G, which solution is saturated at 30°C? (1) 12 grams of KClO3 in 100 grams of water (2) 12 grams of KClO3 in 200 grams of water (3) 30 grams of NaCl in 100 grams of water (4) 30 grams of NaCl in 200 grams of water | 1 | on the line |