Questions | Answer | Explanations | |||||||||||||||
41 The gram formula mass of NH4Cl is
| 3 | NH4Cl=53.5g/mol N=14 H= 4(1)=4 Cl=35.5 | |||||||||||||||
| 42 What is the molarity of a solution that contains 0.50 mole of NaOH in 0.50 liter of solution?
| 1 |
| |||||||||||||||
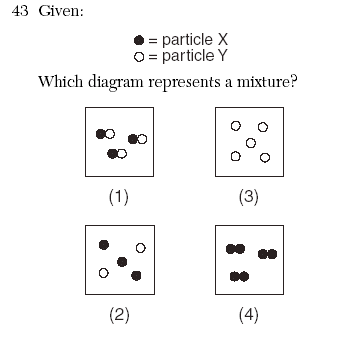 | 2 | 1-compound 2-mixture of elements 3-element 4-diatomic element | |||||||||||||||
44 Which process is accompanied by a decrease in entropy?
| 2 |
| |||||||||||||||
| 45 If 5.0 milliliters of a 0.20 M HCl solution is required to neutralize exactly 10. milliliters of NaOH, what is the concentration of the base?
| 1 |
| |||||||||||||||
| 46 Exactly how much time must elapse before 16 grams of potassium-42 decays, leaving 2 grams of the original isotope?
| 3 | 16->8->4->3 = 3 (1/2 lives) x12.4h | |||||||||||||||
47 Which mass measurement contains four significant figures?
| 3 |
| |||||||||||||||
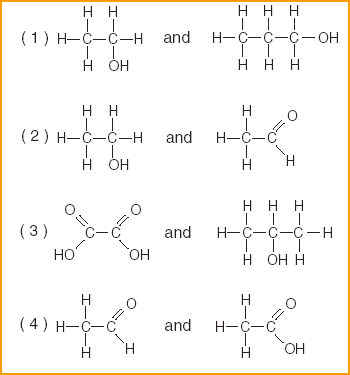
| 48 Which pair of compounds are alcohols?
| 1 | Look for -OH and no other functional groups | |||||||||||||||
49 The process of joining many small molecules into larger molecules is called
| 2 | Definition-polymerization | |||||||||||||||
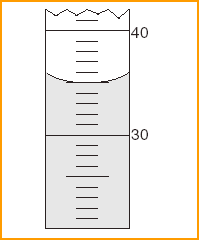
What is the reading of the meniscus?
| 1 | estimate the last decimal place
| |||||||||||||||