Question
Answer
Explanation
11 What is represented by the dots in a Lewis electron-dot diagram of
an atom of an element in Period 2 of the Periodic Table?
(1) the number of neutrons in the atom
(2) the number of protons in the atom
(3) the number of valence electrons in the atom
(4) the total number of electrons in the atom
3
Dots valence electrons
Symbol =kernal –nucleus inner electrons
12 Which type of chemical bond is formed between two atoms of bromine?
(1) metallic (3) ionic
(2) hydrogen (4) covalent
4
Br2 2 nonmetal2s bond covalently
13 Which of these formulas contains the most polar bond?
(1) H–Br (3) H–F
(2) H–Cl (4) H–I
3
Most polar highest electronegativity difference
14 According to Table F, which of these salts is least soluble in water?
(1) LiCl (3) FeCl2
(2) RbCl (4) PbCl2
4
PbCl2 is insoluble
15 Which of these terms refers to matter that could be heterogeneous?
(1) element (3) compound
(2) mixture (4) solution
2
Mixtures can be heterogeneous
16 In which material are the particles arranged in a regular geometric pattern?
(1) CO2 g) (3) H2O(ℓ)
(2) NaCl(aq) (4) C12 H22 O11(s)
4
Definition of a solid
17 Which change is exothermic?
(1) freezing of water
(2) melting of iron
(3) vaporization of ethanol
(4) sublimation of iodine
1
S | L | G |
1 S<-----L | ||
2 S----->L | ||
3 L------>G | ||
4 S--------------->G | ||
18 Which type of change must occur to form a compound?
(1) chemical (3) nuclear
(2) physical (4) phase
1
definition
19 Which formula correctly represents the composition of iron (III) oxide?
(1) FeO3 (3) Fe3O
(2) Fe2O3 (4) Fe3O2
2
Roman numerals are the charge of the metal
Fe=3+ O=2- common multiple 6.
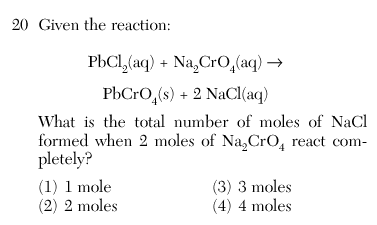
4
2 moles o Na2CrO4 is to 1
as 4 moles of NaCl is
to 2
Use the coefficients to solve this proportion