Question
Answer
Explanation
41 The freezing point of bromine is
(1) 539°C (3) 7°C
(2) –539°C (4) –7°C
4
Table S has melting points=freezing point. 267K=-7C
42 Hexane (C 6 H 14 ) and water do not form a solution. Which statement
explains this phenomenon?
(1) Hexane is polar and water is nonpolar.
(2) Hexane is ionic and water is polar.
(3) Hexane is nonpolar and water is polar.
(4) Hexane is nonpolar and water is ionic.
3
Water is polar
Water is polar
Water is polar
Water is polar
Water is polar
Like dissolves like
Hexane is nonpolar
43 The potential energy diagram below represents a reaction. 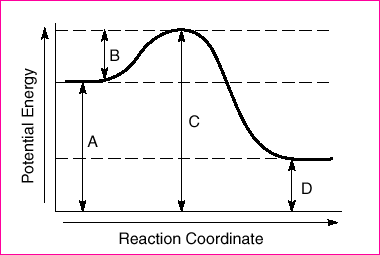 Which arrow represents the activation energy of the forward reaction?
Which arrow represents the activation energy of the forward reaction?
(1) A (3) C
(2) B (4) D
2
Energy to start the reaction
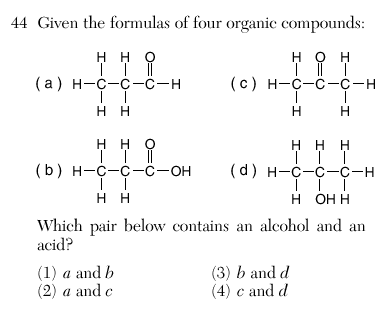
3
-COOH = acid
-OH= alcohol
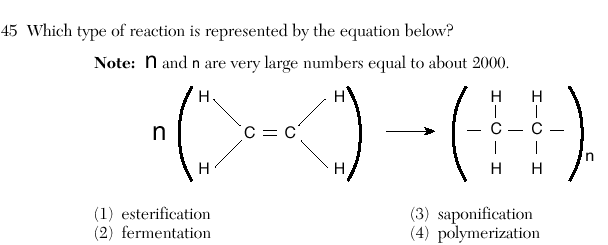
4
polymerization
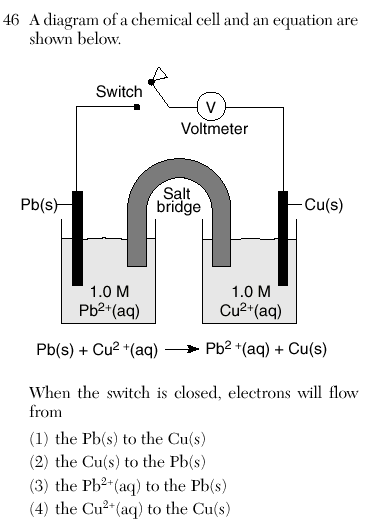
1
Table J-follow the arrow
Oxidation to reduction
Anode to cathode
More reactive to less reactive
47 Which ion has the same electron configuration as an atom of He?
(1) H– (3) Na+
(2) O2– (4) Ca2+
1
H- has 2 electrons also
48 A student was given four unknown solutions. Each solution was
checked for conductivity and tested with phenolphthalein. The result4s
are shown in the data table below.
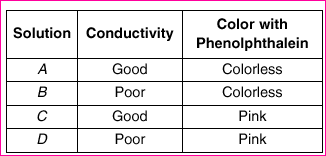
Based on the data table, which unknown solution could be 0.1 M NaOH?
(1) A (3) C
(2) B (4) D
3
NaOH base, so it conducts electricity well and turns pht. pink
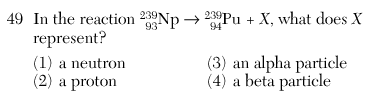
4
Beta particle
50 As carbon dioxide sublimes, its entropy
(1) decreases (2) increases (3) remains the same
2
Solid to gas is an increase in randomness