Question
Answer
Explanation
21 Which hydrocarbon is saturated?
(1) propene (3) butene
(2) ethyne (4) heptane
4
Saturated = all single bonds
alkanes
22 Which statement correctly describes an endothermic chemical reaction?
(1) The products have higher potential energy than the reactants,
and the ΔH is negative.
(2) The products have higher potential energy than the reactants, and
the ΔH is positive.
(3) The products have lower potential energy than the reactants, and
the ΔH is negative.
(4) The products have lower potential energy than the reactants, and
the ΔH is positive.
2
Endothermic=energy in products have more energy than reactans
ΔH is positive
23 At standard pressure when NaCl is added to water, the solution will
have a
(1) higher freezing point and a lower boiling point than water
(2) higher freezing point and a higher boiling point than water
(3) lower freezing point and a higher boiling point than water
(4) lower freezing point and a lower boiling point than water
3
Boiling point elevation
Freezing point depression
24 Which element has atoms that can form single, double, and triple
covalent bonds with other atoms of the same element?
(1) hydrogen (3) fluorine
(2) oxygen (4) carbon
4
Definition of how carbon bonds
25 Which compound is an isomer of pentane?
(1) butane (3) methyl butane
(2) propane (4) methyl propane
3
Must have 5 carbons
26 In which substance does chlorine have an oxidation number of +1?
(1) Cl2 (3) HClO
(2) HCl (4) HClO2
3
HClO
H +1
Cl +1
O -2
27 Which statement is true for any electrochemical cell?
(1) Oxidation occurs at the anode, only.
(2) Reduction occurs at the anode, only.
(3) Oxidation occurs at both the anode and the cathode.
(4) Reduction occurs at both the anode and the cathode.
1
An ox red cat
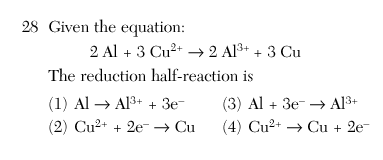
Reduction charge goes down and the electrons are on the more + side
29 Which 0.1 M solution contains an electrolyte?
(1) C6H12O6 (aq) (3) CH3 OH(aq)
(2) CH3 COOH(aq) (4) CH3 OCH3 (aq)
Electrolytes
Acid bases salts
Never CH3OH (not a base but an alcohol)
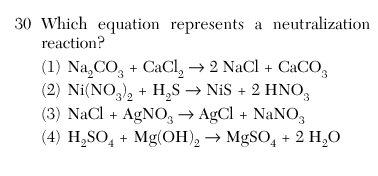
Acid and base makes salt and water