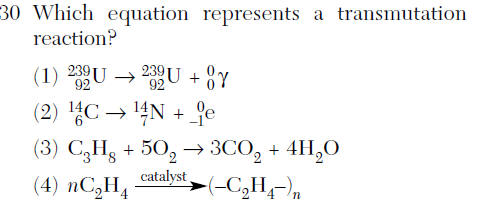Questions
(1) carbon (3) oxygen
(2) nitrogen (4) fluorine
carbon does this
(1) C2H6 (3) C5H8
(2) C3H8 (4) C6H14
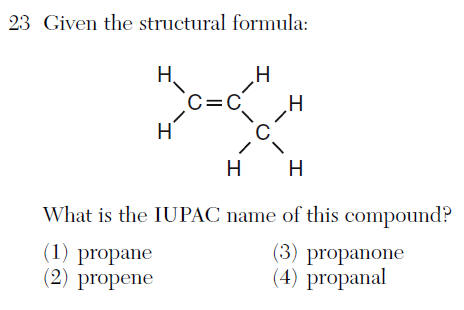
(1) +1 (3) +3
(2) +2 (4) +4
so N= +3 they have to add up to zero
(1) formula masses
(2) molecular formulas
(3) empirical formulas
(4) structural formulas
(1) at the cathode in both an electrolytic cell and a voltaic cell
(2) at the cathode in an electrolytic cell and at the anode in a voltaic cell
(3) at the anode in both an electrolytic cell and a voltaic cell
(4) at the anode in an electrolytic cell and at the cathode in a voltaic cell
Anode Oxidation in either cell
(1) CH3OCH3 (3) CH3COOH
(2) CH3OH (4) C2H5CHO
3 organic acid
only positive ion in the solution is
(1) H+ (3) Na+
(2) Li+ (4) K+
(1) 1.91 days and alpha decay
(2) 1.91 days and beta decay
(3) 3.82 days and alpha decay
(4) 3.82 days and beta decay