Questions | Answer | Explanations |
41 According to Reference Table G, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H2O at
10°C?
(1) KI (3) NaNO3
(2) KNO3 (4) NaCl | 1 | 80g is below KI line at 10°C |
42 What is the concentration of a solution, in parts per million, if 0.02 gram of Na3PO4 is dissolved in 1000 grams of water?
(1) 20 ppm (3) 0.2 ppm
(2) 2 ppm (4) 0.02 ppm | 1 | ppm=(0.02/1000.02) *1,000,000=20ppm |
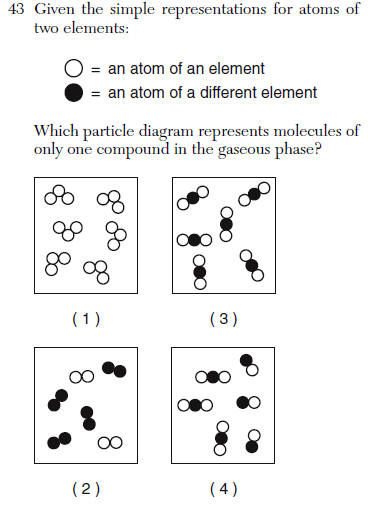 | 3 | compounds have more than one element attached and it is only that molecule in the box |
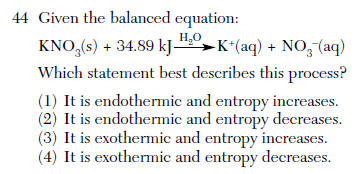 | 1 | endothermic (energy (kj) is on the left of the arrow) s--> aq entropy (randomness) increases |
45 A 1.0-gram piece of zinc reacts with 5 milliliters of HCl(aq). Which of these conditions of concentration and temperature would produce the
greatest rate of reaction?
(1) 1.0 M HCl(aq) at 20.°C
(2) 1.0 M HCl(aq) at at 40.°C
(3) 2.0 M HCl(aq) at at 20.°C
(4) 2.0 M HCl(aq) at at 40.°C | 4 | highest concentration and high temperature means more collisions |
46 At STP, fluorine is a gas and iodine is a solid. This observation can be explained by the fact that fluorine has
(1) weaker intermolecular forces of attraction than iodine
(2) stronger intermolecular forces of attraction than iodine
(3) lower average kinetic energy than iodine
(4) higher average kinetic energy than iodine | 1 | solids have stronger IMFs than gases |
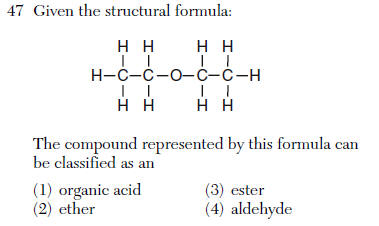 | 2 | -O- is an ether see Table R |
48 Sulfuric acid, H2SO4(aq), can be used to neutralize barium hydroxide, Ba(OH)2(aq). What is the formula for the salt produced by this neutralization?
(1) BaS (3) BaSO3
(2) BaSO2 (4) BaSO4 | 4 | Salt is Barium Sulfate-->BaSO4 |
49 Given the balanced ionic equation:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Which equation represents the oxidation half reaction?
(1) Zn(s) + 2e– → Zn2+(aq)
(2) Zn(s) → Zn2+(aq) + 2e–
(3) Cu2+(aq) → Cu(s) + 2e–
(4) Cu2+(aq) + 2e– → Cu(s) | 2 | charge goes up electrons are on the more positive side |
50 In which solution will thymol blue indicator appear blue?
(1) 0.1 M CH3COOH (3) 0.1 M HCl
(2) 0.1 M KOH (4) 0.1 M H2SO4 | 2 | means it is basic look for OH- |


