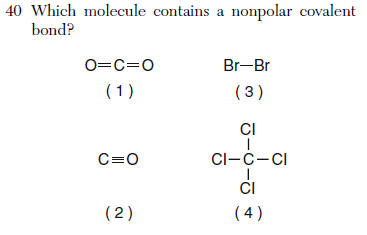
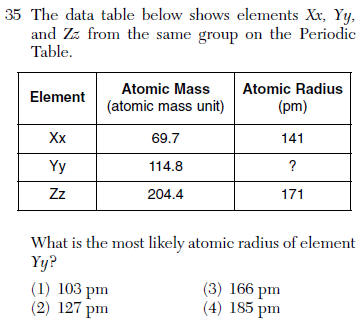
Questions
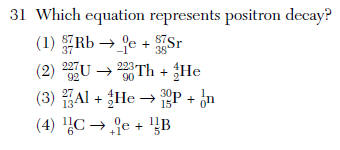
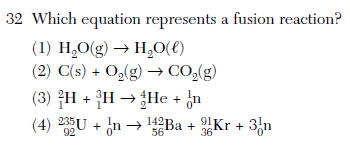
(1) decreases
(2) increases
(3) remains the same
(1) O2– (3) Se2–
(2) Si (4) Ar
same as Ar
(1) sodium oxide
(2) sodium chloride
(3) magnesium oxide
(4) magnesium chloride
(1) CHO (3) C6H12O6
(2) CH2O (4) C12H24O12
compounds is the least soluble in water?
(1) K2CO3 (3) Ca3(PO4)2
(2) KC2H3O2 (4) Ca(NO3)2
the magnesium?
(1) 24.3 g (3) 70.9 g
(2) 48.5 g (4) 142 g