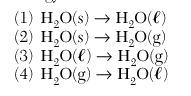Questions | Answer | Explanations | |||||||||||||||
11 Which type or types of change, if any, can reach equilibrium?
| 3 | both | |||||||||||||||
| 12 An increase in the average kinetic energy of a sample of copper atoms occurs with an increase in
| 2 | definition temperature- average kinetic energy | |||||||||||||||
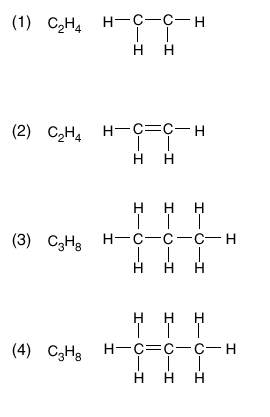
| 13 The empirical formula of a compound is CH2 . Which molecular formula is correctly paired with a structural formula for this compound?
| 2 | carbon must have 4 bonds formula is a multiple of CH2 | |||||||||||||||
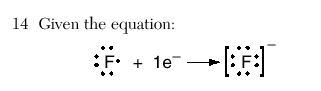 This equation represents the formation of a
| 2 | gaining an electron will make the new ion bigger than that of the original atom | |||||||||||||||
15 The high electrical conductivity of metals is primarily due to
| 3 | metal- a lattice of positive nuclei immersed in a sea of mobile electrons | |||||||||||||||
16 One similarity between all mixtures and compounds is that both
| 4 | mixtures- homo or heterogeneous compounds-homogenous only (pure) | |||||||||||||||
| 17 Which phase change results in the release of energy? | 4 |
| |||||||||||||||
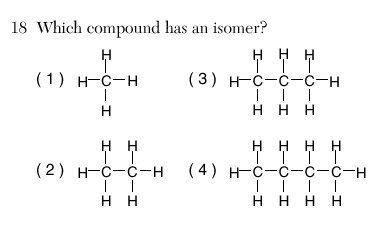 | 4 | there must be 4 carbons to have isomers (butane) | |||||||||||||||
19 What occurs when NaCl(s) is added to water?
| 1 | When a solute is added to water the boiling point increases and the freexing point decreases. This is proportional to the amount of particles in the solution (remember ionic subs. break up) | |||||||||||||||
20 Which radioisotope is a beta emitter?
| 1 | Use Table N- Decay Mode | |||||||||||||||