Questions | Answer | Explanations |
| 21 When a mixture of water, sand, and salt is filtered, what passes through the filter paper?
| 3 | the salt will dissolve in the water and pass through the filter paper |
| 22 A hydrate is a compound that includes water molecules within its crystal structure. During an experiment to determine the percent by mass of water in a hydrated crystal, a student found the mass of the hydrated crystal to be 4.10 grams. After heating to constant mass, the mass was 3.70 grams. What is the percent by mass of water in this crystal?
| 3 | mass water= 4.10-3.70=0.40g (0.40/4.10)*100% |
23 Which of these 1 M solutions will have the highest pH?
| 1 | high pH is a base NaOH (OH-) is a base |
| 24 Which physical property makes it possible to separate the components of crude oil by means of distillation?
| 4 | distillation use boiling point differences |
25 In saturated hydrocarbons, carbon atoms are bonded to each other by
| 1 | saturated hydrocarbons-all single bonds-alkanes |
| 26 Which formula correctly represents the product of an addition reaction between ethene and chlorine?
| 3 | C2H4 + Cl2 -->C2 H4 Cl2 |
27 When a neutral atom undergoes oxidation, the atomís oxidation state
| 4 | ox # increase-loses electrons |
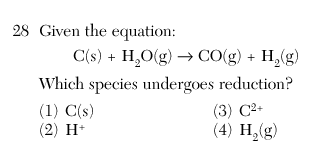 | 2 | 2H+ --> H2o |
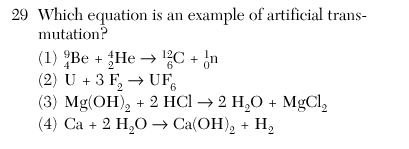 | 1 | 2 reactants and the isotopic notations |
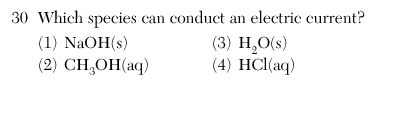 | 4 | acid base salt --> aqueous |