41 Which phase change represents a decrease in entropy?
| 2 |
| |||||||||||||||
| 42 Given the equation: 2C2H2 (g) + 5O2 (g) → 4 CO2 (g) + 2H2O(g) How many moles of oxygen are required to react completely with 1.0 mole of C2 H2 ?
| 1 |
| |||||||||||||||
| 43 A student intended to make a salt solution with a concentration of 10.0 grams of solute per liter of solution. When the student’s solution was analyzed, it was found to contain 8.90 grams of solute per liter of solution. What was the percent error in the concentration of the solution?
| 3 |
| |||||||||||||||
44 What is the molarity of a solution of NaOH if 2 liters of the solution contains 4 moles of NaOH?
| 2 |
| |||||||||||||||
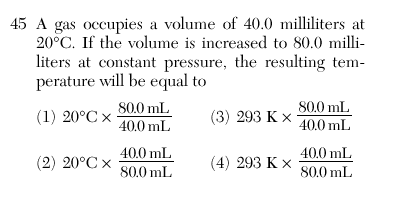 | 3 | Temperature must be in Kelvin
| |||||||||||||||
| 6 According to Reference Table J, which of these metals will react most readily with 1.0 M HCl to produce H2(g)?
| 2 | The higher up on table J | |||||||||||||||
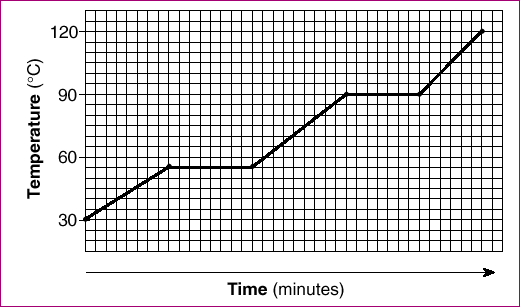
| 47 The graph below represents the heating curve of a substance that starts as a solid below its freezing point.
What is the melting point of this substance?
| 2 | The lower plateu is the melting/freezing phase change. Look at teh temp. there. The upper plateu should be longer and it represents vaporization (requires more energy), | |||||||||||||||
| 48 Given the unbalanced equation: __Fe2O3 + __CO → __Fe + __CO2 When the equation is correctly balanced using the smallest whole-number coefficients, what is the coefficient of CO?
| 3 | 1:3:2:3 | |||||||||||||||
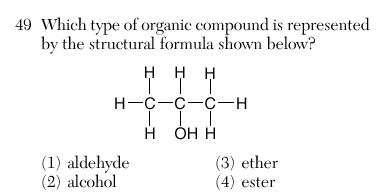 | 2 | alcohol -OH only | |||||||||||||||
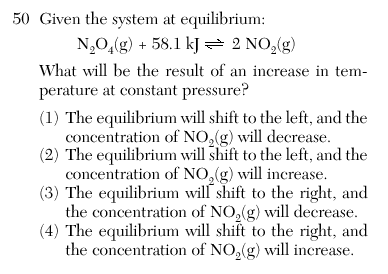 | 4 | Since energy is a reactant it will consume the added energy and shift right, therefore increasing the [NO2]. | |||||||||||||||