Questions | Answer | Explanations |
| 11 Which must be a mixture of substances? (1) solid (3) gas (2) liquid (4) solution | 4 | SOLUTIONS ARE MADE UP OF A SOLUTE AND SOLVENT |
| 12 A bottle of rubbing alcohol contains both 2-propanol and water. These liquids can be separated by the process of distillation because the 2-propanol and water (1) have combined chemically and retain their different boiling points (2) have combined chemically and have the same boiling point (3) have combined physically and retain their different boiling points (4) have combined physically and have the same boiling point | 3 | they are mixed (physical) keep there properties (BP) |
| 13 Compared to pure water, an aqueous solution of calcium chloride has a (1) higher boiling point and higher freezing point (2) higher boiling point and lower freezing point (3) lower boiling point and higher freezing point (4) lower boiling point and lower freezing point | 2 | addition of a solute raises the BP and lowers the FP |
| 14 Under which conditions does a real gas behave most like an ideal gas? (1) at low temperatures and high pressures (2) at low temperatures and low pressures (3) at high temperatures and high pressures (4) at high temperatures and low pressures | 4 | ideal gas are like ideal vacations high temp low pressures |
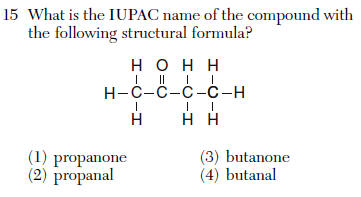 | 3 | But- 4 carbons -an- single bonds -one ketone |
| 16 Which statement best explains the role of a catalyst in a chemical reaction? (1) A catalyst is added as an additional reactant and is consumed but not regenerated. (2) A catalyst limits the amount of reactants used. (3) A catalyst changes the kinds of products produced. (4) A catalyst provides an alternate reaction pathway that requires less activation energy. | 4 | Definition of a catalyst |
| 17 Given the reaction at equilibrium: H2(g) + Br2(g) --> 2 HBr(g) The rate of the forward reaction is (1) greater than the rate of the reverse reaction (2) less than the rate of the reverse reaction (3) equal to the rate of the reverse reaction (4) independent of the rate of the reverse reaction | 3 | Concentrations Constant and the Rates are equal |
| 18 Which statement best explains why most atomic masses on the Periodic Table are decimal numbers? (1) Atomic masses are determined relative to an H–1 standard. (2) Atomic masses are determined relative to an O–16 standard. (3) Atomic masses are a weighted average of the naturally occurring isotopes. (4) Atomic masses are an estimated average of the artificially produced isotopes. | 3 | Definition of Atomic Mass |
| 19 All organic compounds must contain the element (1) phosphorus (3) carbon (2) oxygen (4) nitrogen | 3 | Organic- contains carbon |
| 20 Which of the following compounds has the highest boiling point? (1) H2O (3) H2Se (2) H2S (4) H2Te | 1 | Hydrogen Bonding Question H with F O or N |