Questions | Answer | Explanations |
| 41 What is the Molarity of a solution containing 20 grams of NaOH in 500 milliliters of solution? (1) 1 M (3) 0.04 M (2) 2 M (4) 0.5 M | 1 | mole/Liters moles=20g/40g=0.5mol 500mL is 0.5L M=0.5mol/0.5L= 1M |
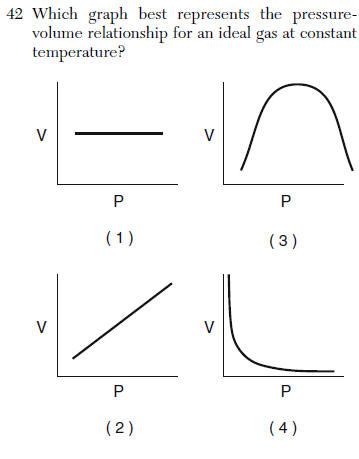 | 4 | indirect relationship |
| 43 Given the equation: C2H6 + Cl2 --> C2H5Cl + HCl This reaction is best described as (1) addition involving a saturated hydrocarbon (2) addition involving an unsaturated hydrocarbon (3) substitution involving a saturated hydrocarbon (4) substitution involving an unsaturated hydrocarbon | 3 | Substitution Saturated |
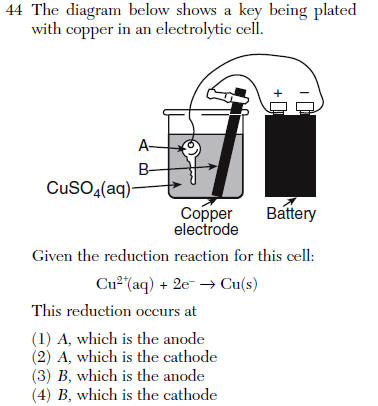 | 2 | Cu2+ is reduced to Cu on the key
Red Cat |
| 45 A student neutralized 16.4 milliliters of HCl by adding 12.7 milliliters of 0.620 M KOH. What was the molarity of the HCl acid? (1) 0.168 M (3) 0.620 M (2) 0.480 M (4) 0.801 M | 2 | MV=MV M(16.4mL)= (0.620M)(12.7mL) M=0.480M |
| 46 Nuclear fusion differs from nuclear fission because nuclear fusion reactions (1) form heavier isotopes from lighter isotopes (2) form lighter isotopes from heavier isotopes (3) convert mass to energy (4) convert energy to mass | 2 | H + H--> He fusion (light atoms) U + n--> Ba + Kr +3n fission (heavy atoms) |
| 47 After 32 days, 5 milligrams of an 80-milligram sample of a radioactive isotope remains unchanged. What is the half-life of this element? (1) 8 days (3) 16 days (2) 2 days (4) 4 days | 1 | 80-->40-->20-->10-->5 4 half lives 32days/4 = 8 days per half life |
| 48 Which electron configuration represents an atom of chlorine in an excited state? (1) 2–8–7 (3) 2–8–6–1 (2) 2–8–8 (4) 2–8–7–1 | 3 | 17 electrons with 1 promoted |
| 49 As each successive element in Group 15 of the Periodic Table is considered in order of increasing atomic number, the atomic radius (1) decreases (2) increases (3) remains the same | 2 | See table S, or atomic snowman o 0 O |
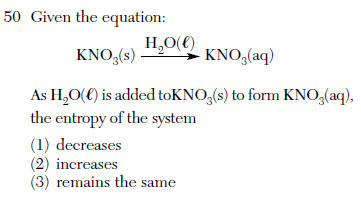 | 2 | adding water causes more KNO3 to dissolve so solid becomes aqueous, entropy increases |