Questions | Answer | Explanations |
| 31 When an atom of phosphorus becomes a phosphide ion (P3–), the radius (1) decreases (2) increases (3) remains the same | 2 | gains electrons is gets bigger |
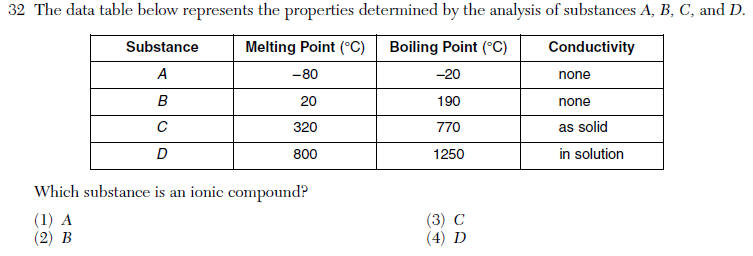 | 4 | high melting point/ boiling pt, conducts electricity in water |
| 33 What is the total number of electrons in a Cr3+ion? (1) 18 (3) 24 (2) 21 (4) 27 | 2 | Cr 24 protons so when it is 3+ it has 21 electrons |
| 34 As the atoms of the Group 17 elements in the ground state are considered from top to bottom, each successive element has (1) the same number of valence electrons and similar chemical properties (2) the same number of valence electrons and identical chemical properties (3) an increasing number of valence electrons and similar chemical properties (4) an increasing number of valence electrons and identical chemical properties | 1 | same group same val. e- and similar properties |
| 35 Which solution when mixed with a drop of bromthymol blue will cause the indicator to change from blue to yellow? (1) 0.1 M HCl (3) 0.1 M CH3OH (2) 0.1 M NH3 (4) 0.1 M NaOH | 1 | it would be acidic Table M, look for a compound starting with H |
| 36 What is the empirical formula of a compound with the molecular formula N2O4? (1) NO (3) N2O (2) NO2 (4) N2O3 | 2 | lowest whole number ratio |
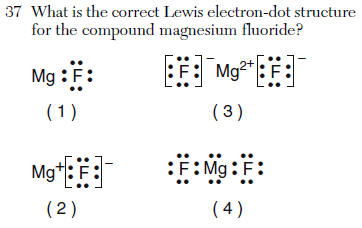 | 3 | it is ionic the Mg has no valance electrons and the Cl has 8, also include the charges |
| 38 Given the reaction: Mg(s) + 2 AgNO3(aq)--> Mg(NO3)2(aq) + 2 Ag(s) Which type of reaction is represented? (1) single replacement (3) synthesis (2) double replacement (4) decomposition | 1 | Mg replaces Ag, single replacement |
| 39 Which equation shows conservation of both mass and charge? (1) Cl2 + Br--> Cl- + Br2 (2) Cu + 2 Ag+ --> Cu2+ + Ag (3) Zn + Cr3+ --> Zn2+ + Cr (4) Ni + Pb2+ --> Ni2+ + Pb | 4 | atoms are balanced (mass) total charges are +2 on both sides |
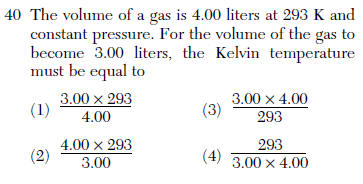 | 1 | V/T =V/T plug in solve for T 4/293 =3/T |