Questions | Answer | Explanations |
| 21 The functional group —COOH is found in (1) esters (3) alcohols (2) aldehydes (4) organic acids | 4 | Reference Table R General Formula |
| 22 Which of these elements is the best conductor of electricity? (1) S (3) Br (2) N (4) Ni | 4 | Ni is a METAL |
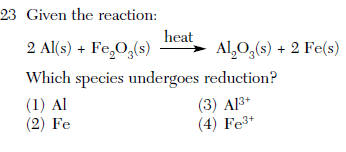 | 4 | Add ox. #s reduction charge goes down Fe3+ --> Feo |
| 24 Which energy transformation occurs when an electrolytic cell is in operation? (1) chemical energy → electrical energy (2) electrical energy → chemical energy (3) light energy → heat energy (4) light energy → chemical energy | 2 | electrolytic requires a power source |
| 25 Which of these pH numbers indicates the highest level of acidity? (1) 5 (3) 10 (2) 8 (4) 12 | 1 | acids have low pH values |
| 26 According to the Arrhenius theory, when a base dissolves in water it produces (1) CO3 2– as the only negative ion in solution (2) OH– as the only negative ion in solution (3) NH4 + as the only positive ion in solution (4) H+ as the only positive ion in solution | 2 | base OH- |
| 27 Which compound is an electrolyte? (1) C6H12O6 (3) CaCl2 (2) CH3OH (4) CCl4 | 3 | acids, bases or salts CaCl2 is a salt |
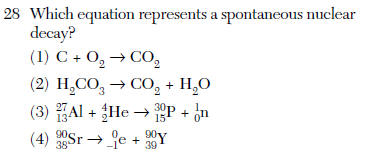 | 4 | 1 reactant aka natural transmutation |
| 29 The stability of an isotope is based on its (1) number of neutrons, only (2) number of protons, only (3) ratio of neutrons to protons (4) ratio of electrons to protons | 3 | found in the nucleus |
| 30 As the temperature of a substance decreases, the average kinetic energy of its particles (1) decreases (2) increases (3) remains the same | 1 | avg. KE is Temperature |