Question
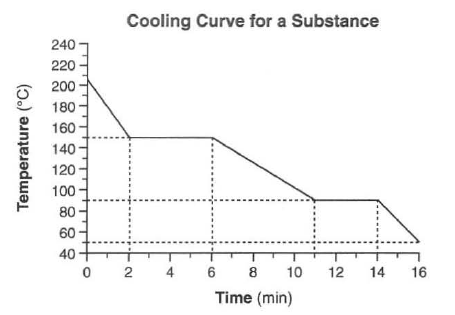
Starting as a gas at 206°C, a sample of a substance is allowed to cool for 16 minutes. This process is represented by the cooling curve below.
55 What is the melting point of this substance? [1]HIGHLIGHT TO SEE THE ANSWER
56 At what time do the particles of this sample have the lowest average kinetic energy? [1] HIGHLIGHT TO SEE THE ANSWER
57 Using the key in your answer booklet, draw two particle diagrams to represent the two phases of the sample at minute 4. Your response must include at least six particles for each diagram. [1]
|
on to Questions 58-59