Questions
31 What is the total number of valence electrons in a germanium atom in the ground state?
(1) 22 (3) 32
(2) 2 (4) 4
last number of the electron configuration on the Periodic table
32 Which element is paired with an excited-state electron configuration for an atom of the element?
(1) Ca: 2-8-8-2 (2) Na: 2-8-2
(3) K: 2-6-8-3 (4) F: 2-8
K should be 2-8-8-1 (ground state)
33 Given the balanced equations representing two chemical reactions:
Cl2
+ 2NaBr ---> 2NaCl + Br22NaCl
---> 2Na + Cl2Which types of chemical reactions are represented
by these equations?(1) single replacement and decomposition
(2) single replacement and double replacement
(3) synthesis and decomposition
(4) synthesis and double replacement
34 An ion that consists of 7 protons, 6 neutrons, and 10 electrons has a net charge of
(I ) 4- (3) 3+
(2) 3- (4) 4+
35 Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?
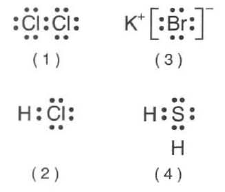
2 of the same element bonded= nonpolar bond
36
Which quantity is equal to 50 kilojoules?(I ) 0.05J (3) 5 x 103J
(2) 500J (4) 5 x 104J
50 x 103 which is 5 x 104
37 Which compound is formed from its elements by an exothermic reaction at 298 K and 101.3 kPa?
(1) C2H4(g) (2) HI(g)
(3) H2O(g) (4) NO2(g)
38 At which temperature is the vapor pressure of ethanol equal to 80. kPa?
(1) 48°C (3) 80. °C
(2) 73°C (4) 101 °C
39 At 25°C, gas in a rigid cylinder with a movable piston has a volume of 145 mL and a pressure of 125 kPa. Then the gas is compressed to a volume of 80. mL. What is the new pressure of the gas if the temperature is held at 25°C?
(1) 69 k
Pa (3) 160 kPa(2) 93 kPa (4) 230 kPa
PV=PV (temperature is constant, so leave it out)
145ml x 125kPa= P x 80.ml
226 kPa=P
230kPa=P (sig figs)
40 A 2400.-gram sample of an aqueous solution contains 0.012 gram of NH3
. What is the concentration of NH3 in the solution, expressed as parts per million?(1) 5.0
ppm (2) 15 ppm (3) 20. ppm (4) 50. ppm(0.012g/2400.g) x 1,000,000=5.0ppm